Acotiamide hydrochloride impurity and preparation method thereof
A technology for acotiamide hydrochloride and impurities, which is applied in the field of new impurities of acotiamide hydrochloride and its preparation, can solve problems such as searching for structural information, achieve high yield and purity, simple post-treatment, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
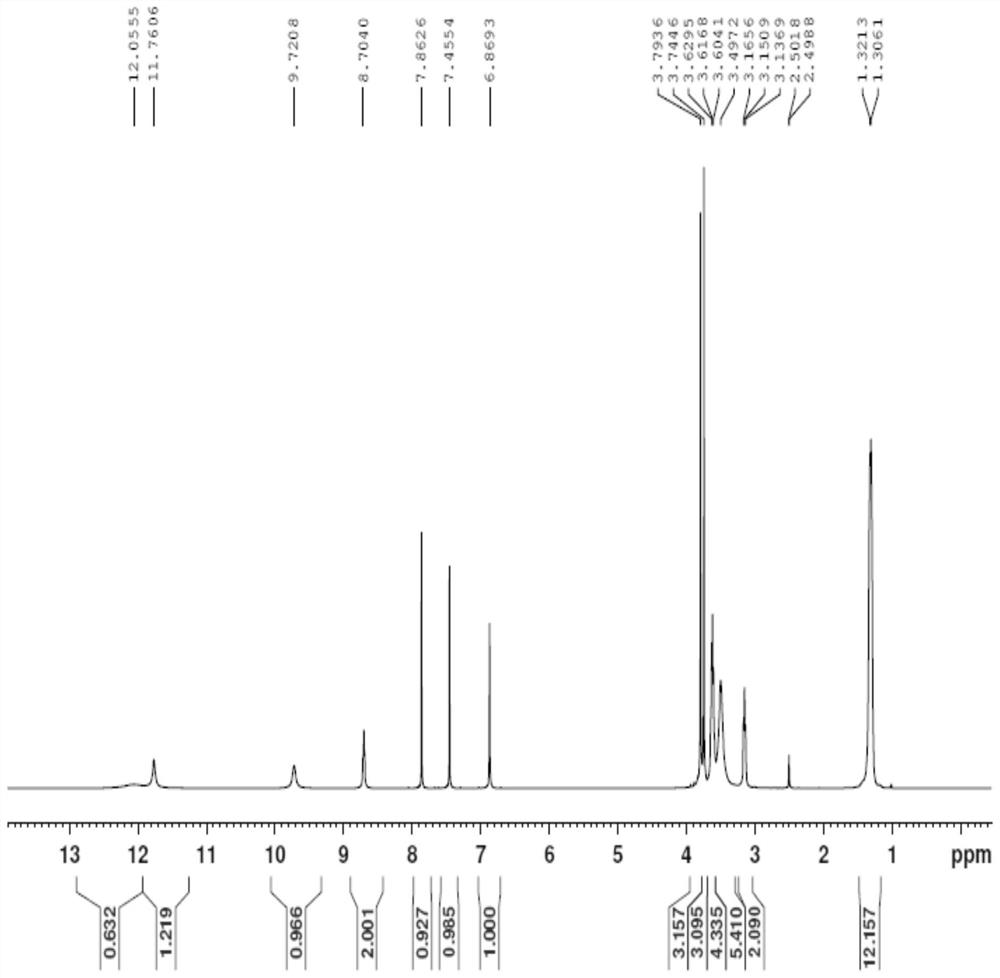
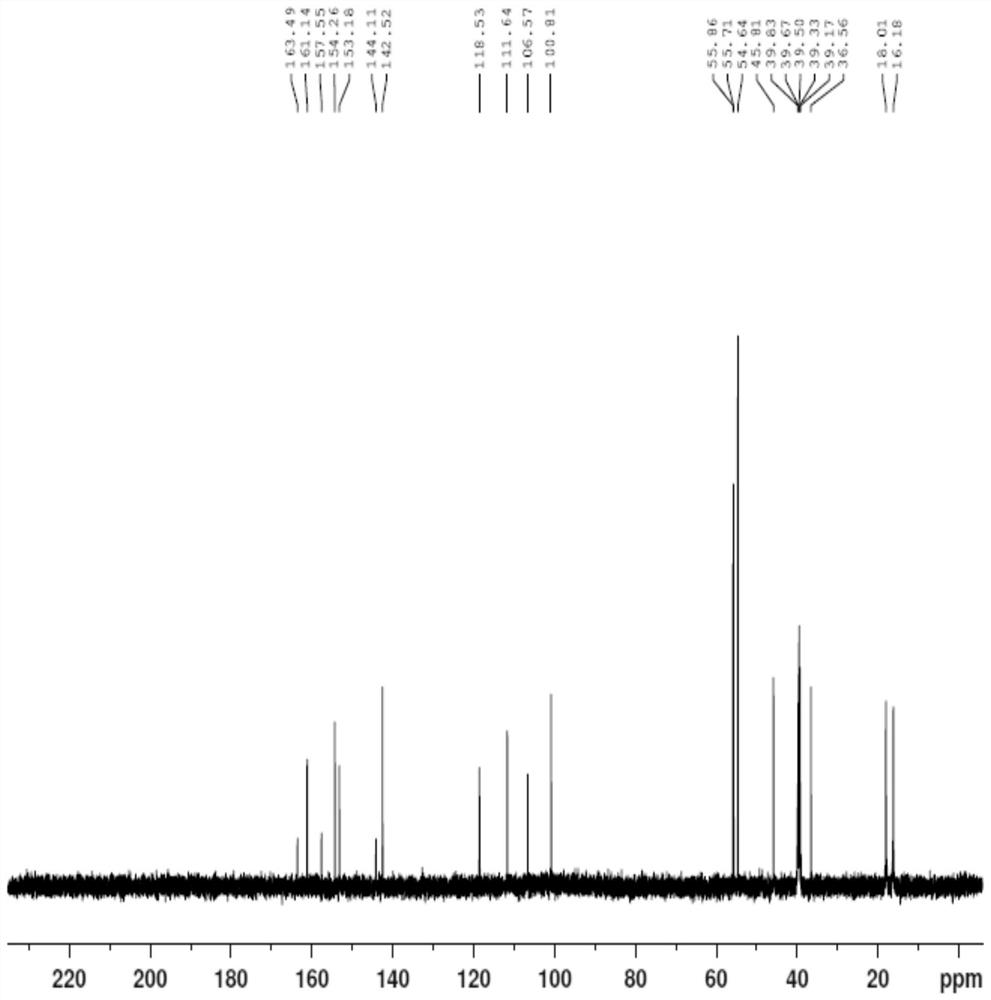
[0056] Example 1: (Z)-S-(2-amino-3-((2-(diisopropylamino)ethyl)amino)-3-oxoprop-1-en-1-yl)(2- Preparation of hydroxy-4,5-dimethoxybenzoyl) carbamate (impurity shown in formula II)
[0057] Add compound IV (10.00g, 22.20mmol) and 60mL of 80% isopropanol aqueous solution into a 250ml reaction flask, stir well and heat up to 60-70°C to completely dissolve the solid. Then, sodium hydroxide (0.44 g, 11.00 mmol) was added to the resulting mixed solution, and the temperature was raised to 70-80° C. while stirring, and the reaction was continued for 48 h while insulated and stirred, and the reaction of compound IV was monitored by TLC to complete. After the reaction is over, stop stirring, lower the temperature to 20-30°C, stir and crystallize for 4 hours, a large amount of solids precipitate, filter with suction, rinse the filter cake with 20mL of purified water, and vacuum the obtained solids under the conditions of 50°C and -0.09MPa After drying, 9.56 g of the compound of formula ...
Embodiment 2
[0059] Example 2: (Z)-2-amino-3-(((2-hydroxyl-4,5-dimethoxybenzoyl)carbamoyl)thio)acrylic acid (impurity shown in formula III) preparation
[0060] Add compound V (10.00g, 30.83mmol) and 100mL of 80% isopropanol aqueous solution into a 250ml reaction flask, stir well and heat up to 50-60°C to completely dissolve the solid. Then, sodium hydroxide (0.25 g, 6.25 mmol) was added to the resulting mixed solution, and the temperature was raised to 70-80° C. while stirring, and the reaction was continued for 50 h while insulated and stirred, and the reaction of compound V was monitored by TLC to complete. After the reaction is over, stop stirring, lower the temperature to 20-30°C, stir and crystallize for 4 hours, a large amount of solids precipitate, filter with suction, rinse the filter cake with 20mL of purified water, and vacuum the obtained solids under the conditions of 50°C and -0.09MPa After drying, 9.82 g of the compound of formula III was obtained, with a yield of 93.03% an...
Embodiment 3
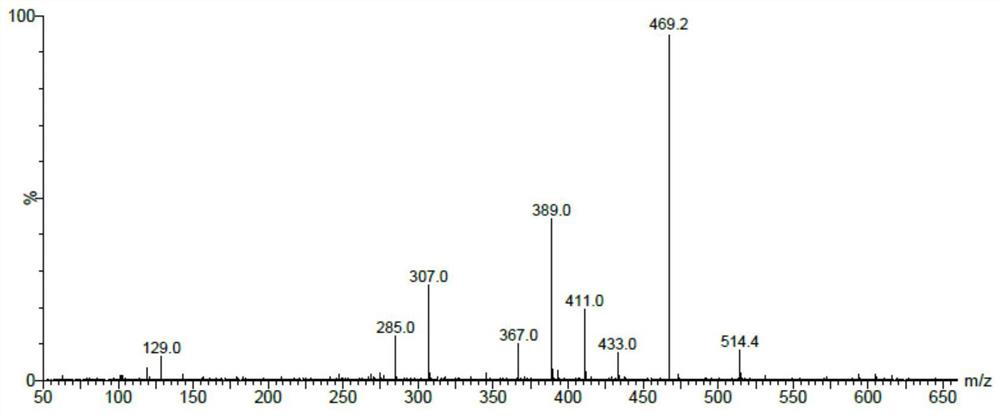
[0062] Embodiment 3: the detection of acotiamide hydrochloride finished product
[0063] Get an appropriate amount of acotiamide hydrochloride finished product, accurately weigh, dissolve in an appropriate amount with diluent (mobile phase A-mobile phase B (85:15)), and dilute to make the solution that contains about 0.5mg in every 1ml, as test product solution. With reference to the high performance liquid chromatography (Chinese Pharmacopoeia 2015 edition four general rules 0512) test, use pentafluorophenyl bonded silica gel as filler (250 × 4.6mm, 5 μm); use 20nmol / L ammonium formate solution (ammonium formate 1.26g, Add 1000ml of water to dissolve, adjust the pH value to 3.0 with formic acid) as mobile phase A, and acetonitrile as mobile phase B, and carry out gradient elution as shown in the table below; the detection wavelength is 280nm, and the column temperature is 30°C. Precisely measure 20 μl of the test solution, inject it into the liquid mass spectrometry detector...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com