Heterologous expression of glutamine transaminase and application thereof
A glutamine and transaminase technology, applied in the direction of acyltransferase, application, transferase, etc., can solve the problems of low expression, difficulty in high-efficiency expression of TGase, and no catalytic activity of inclusion bodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment one: the acquisition of TGase gene
[0015] extract Streptomyces Genomic DNA of sp. TYQ1024 was sequenced for whole genome sequence. The obtained genome data is analyzed to obtain the transglutaminase gene TGase.
Embodiment 2
[0016] Embodiment two: the heterologous expression of TGase gene
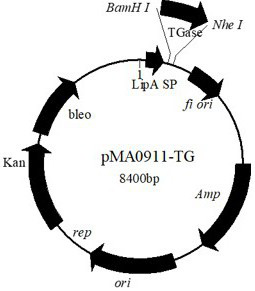
[0017] Design specific primers to amplify TGase gene, upstream primer (5'-CGC GGATCC TCGCCACCGGCAGTGGCAGTGGCA-3'), downstream primer (5'-CTA GCTAGC TCACGGCCAGCCCTGTGTCA-3'), the TGase gene was obtained by PCR. Perform TGase gene and plasmid pMA LipA Bam H and Nhe Digest and ligate. Then, the ligation product was transformed into E. coli DH5α, the recombinant plasmid pMA LipA-TG was extracted and transformed into B. subtilis SCK6.
Embodiment 3
[0018] Embodiment three: Recombinant Bacillus subtilis engineered bacterium culture condition
[0019] recombinant bacteria B. subtilis SCK6 / pMA LipA-TG was inoculated into 100 mL TB basal medium containing 50 µg / mL Kanamycin, cultured on a shaker at 37°C for 16 hours, and inoculated at a 3% inoculum size into the fermentation culture containing 50 µg / mL Kan Medium (medium composition: glucose 15 g / L, soybean peptone 20 g / L, urea 20 g / L, potassium dihydrogen phosphate 2.31 g / L, dipotassium hydrogen phosphate 0.54 g / L, pH 6.5), 37° C, cultured on a shaker at 220 rpm for 64 hours; centrifuged, collected the fermentation supernatant, and measured the TGase activity in the supernatant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com