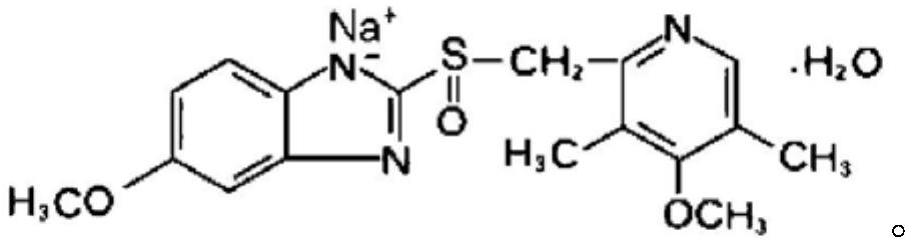
Omeprazole sodium for injection and preparation method thereof
A technology for omeprazole sodium and injection, applied in the field of preparation of sterile powder injection, can solve the problems of polymerization, discoloration, impurity polymerization, discoloration, etc., and achieve the effects of improving plumpness, improving looseness, and reducing the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 A kind of preparation method of omeprazole sodium for injection
[0035] Present embodiment is a kind of preparation method of omeprazole sodium for injection, and concrete preparation process comprises the following steps of carrying out successively:
[0036] Take water for injection, boil it and let it cool for later use;
[0037] Take 199.63g glycine and add water for injection to prepare 13.3L glycine aqueous solution, which is 1mol / L glycine aqueous solution;
[0038] Take 144.18g of disodium hydrogen phosphate (dihydrate) and add water for injection to prepare 16.2L of disodium hydrogen phosphate aqueous solution, which is 0.2mol / L disodium hydrogen phosphate aqueous solution;
[0039] Take 82g of sodium hydroxide and add water for injection to prepare 20.5L of sodium hydroxide aqueous solution, which is 0.1mol / L sodium hydroxide aqueous solution.
[0040] Take glycine aqueous solution, disodium hydrogen phosphate aqueous solution and sodium hydrox...
Embodiment 2~6
[0050] The preparation method of embodiment 2~6 injection omeprazole sodium
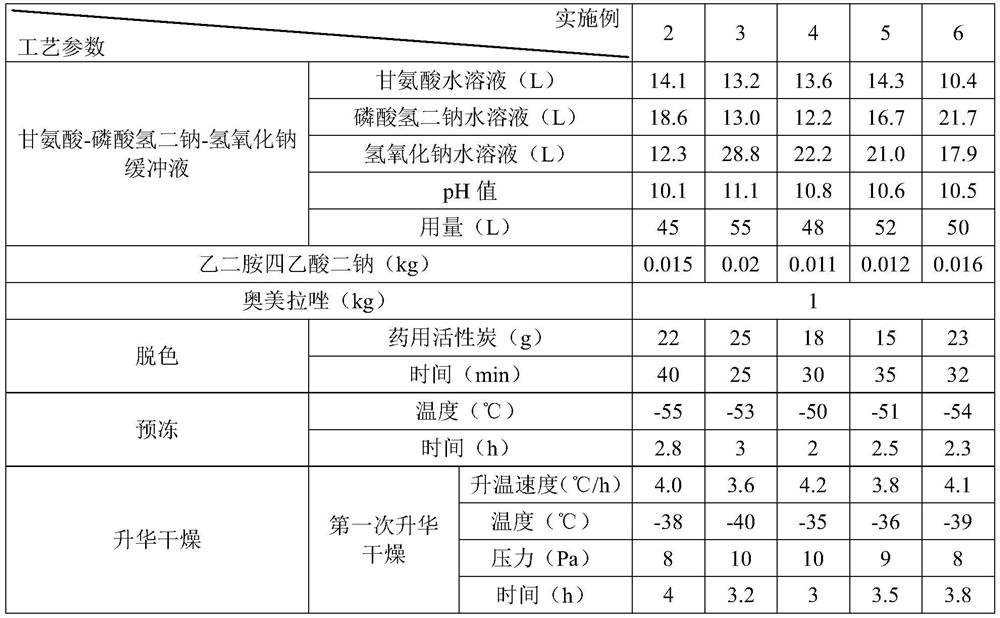
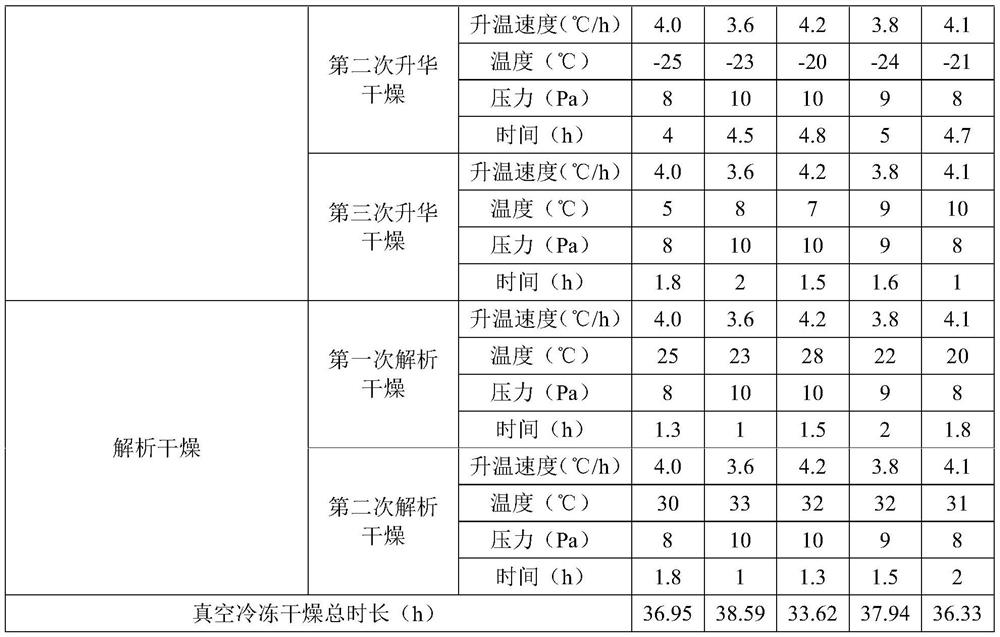
[0051] Embodiments 2 to 6 are respectively a preparation method of omeprazole sodium for injection, and their steps are basically the same as in Example 1, the difference is only in the amount of raw materials and process parameters, see Table 1 for details:
[0052] List of each technological parameter in table 1 embodiment 2~6
[0053]
[0054]
[0055] The content of the other parts of Examples 2-6 is the same as that of Example 1.
[0056] The omeprazole sodium for injection prepared in Examples 2-6 has good stability, high main drug content, less impurities and excellent resolubility.
experiment example 1
[0057] Performance measurement of experimental example 1 omeprazole sodium for injection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com