Standardized vein nutrient solution formula for newborns with low birth weight and preparation device
A low-birth-weight, neonatal technology, applied in pharmaceutical formulations, packaging, packaged food, etc., can solve the problems of prone to polluting neonatal wards, waste of human resources and drugs, and high requirements for preparation processes, achieving medical management efficiency and efficiency. The effect of quality improvement, aseptic operation, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
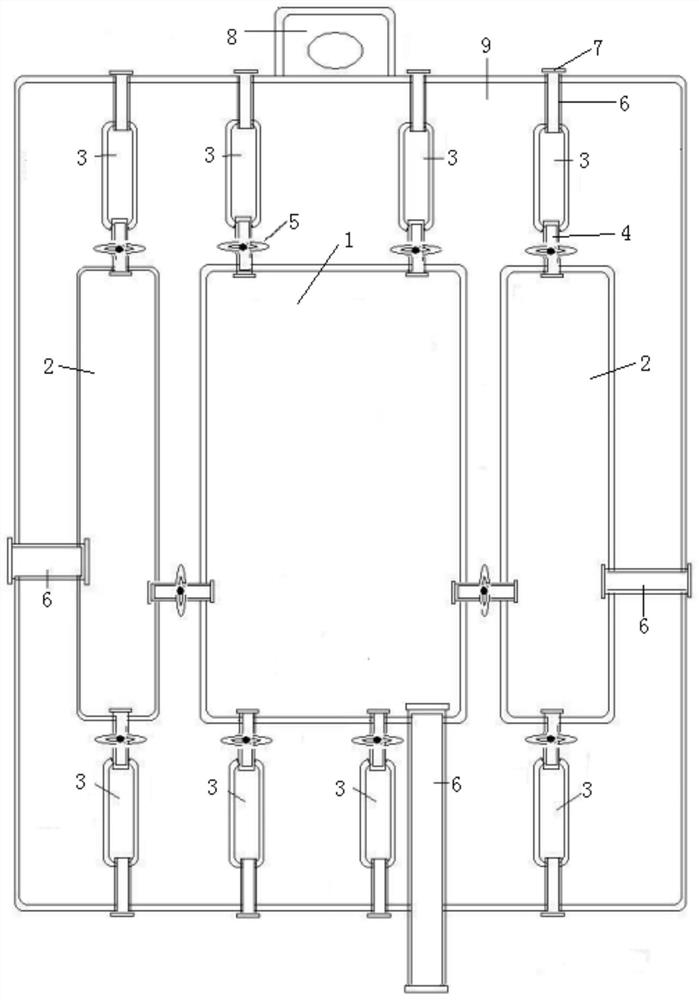
Image
Examples
Embodiment 1
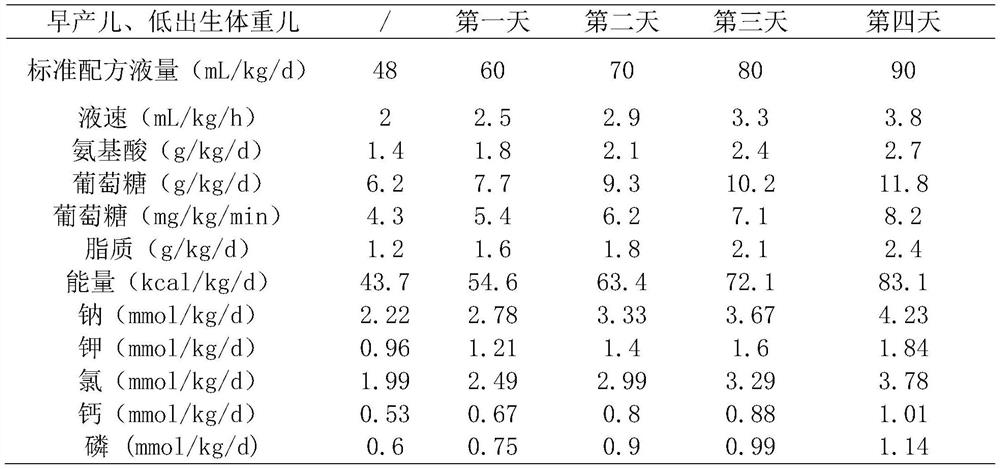
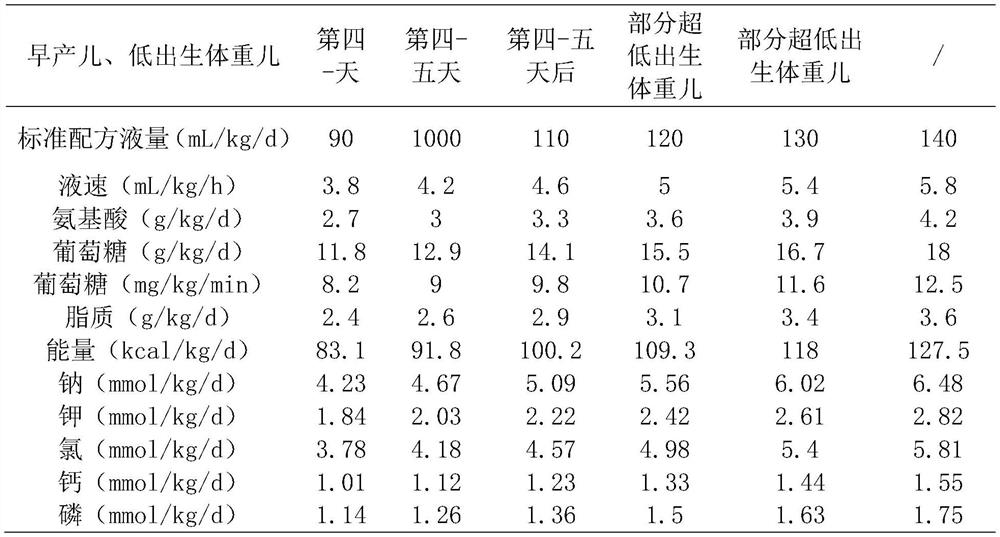
[0039] A standardized parenteral nutrition solution for low birth weight neonates, in terms of 200mL, its components are as follows:
[0040] Shui Le Vita 0.15 sticks, 10% sodium chloride 2mL, 15% potassium chloride 1.5mL, Griffith 2mL, 20% fat emulsion 20mL, 6% children's compound amino acid injection 19AA 75mL, 50% glucose 10.5mL, 10% glucose 80mL, Vitalipit 1.5mL, various trace elements injection I 1.5mL, 10% calcium gluconate injection 5mL, levocarnitine 1mL.
Embodiment 2
[0042] A standardized parenteral nutrition solution for low birth weight neonates, in terms of 200mL, its components are as follows:
[0043] Shui Le Vita 0.2 sticks, 10% Sodium Chloride 3mL, 15% Potassium Chloride 3mL, Griffith 5mL, 20% Fat Emulsion 24mL, 6% Pediatric Compound Amino Acid Injection 19AA 90mL, 50% Glucose 30.5mL, 10 % Glucose 24mL, Vitalipit 2mL, Multiple Trace Elements Injection I 2mL, 10% Calcium Gluconate Injection 15mL, Levocarnitine 1.5mL.
Embodiment 3
[0045] A standardized parenteral nutrition solution for low birth weight neonates, in terms of 200mL, its components are as follows:
[0046]Shui Le Vita 0.2 sticks, 10% sodium chloride 2.5mL, 15% potassium chloride 2mL, Griffith 2.5mL, 20% fat emulsion 26mL, 6% children's compound amino acid injection 19AA 100mL, 50% glucose 51.5mL , Vitalipit 2mL, multiple trace element injection I 2mL, levocarnitine 1.5mL, 10% calcium gluconate injection 10mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com