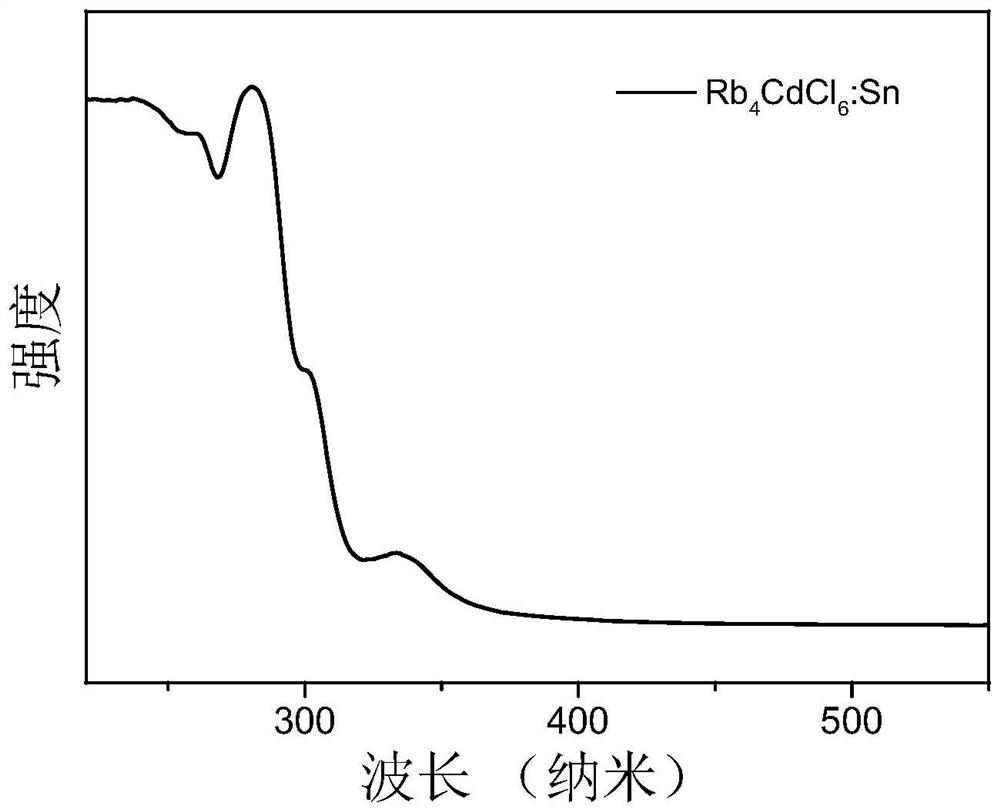
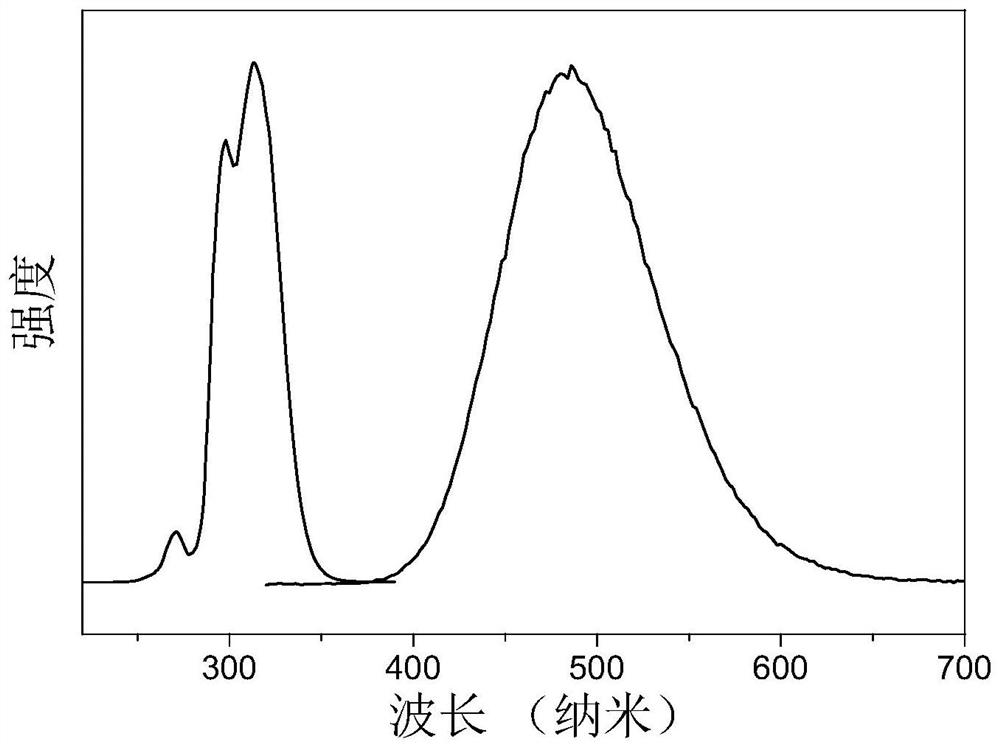
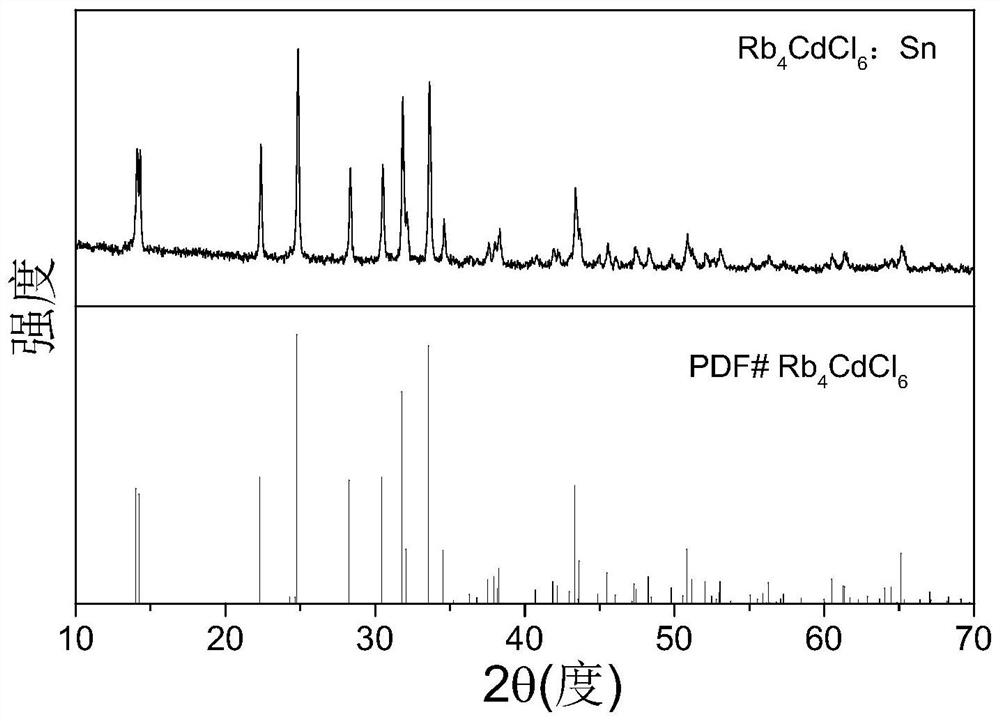
Ternary metal halide with ultrahigh fluorescence efficiency and preparation method
A ternary metal and halide technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of low luminous efficiency and difficult application in luminescent fields, and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Weigh 4mmol rubidium chloride, 0.9mmol anhydrous cadmium chloride, and 0.1mmol stannous chloride in the glove box, pour them into a medium mortar and grind them thoroughly, mix them evenly and transfer them to a hydrothermal reaction kettle, the above process All performed in a glove box. Next, add 4 mL of concentrated hydrochloric acid, 200 μL of hypophosphorous acid, and 0.1 mmol of sodium citrate into the reaction kettle. Seal the reaction kettle and put it into a tube furnace. Set the program to raise the temperature from room temperature to 170 ° C over 180 minutes, and maintain at this temperature 600min, then slowly cool down to room temperature at 5°C / h to obtain powdered Rb 4 CdCl 6 Crystals, the obtained crystals were transferred to a Buchner funnel covered with filter paper for suction filtration, and the crystals were continuously washed with isopropanol during the suction filtration process. Immediately afterwards, immerse the crystal in 3mL ethanol solut...
Embodiment 2
[0023] The consumption of the concentrated hydrochloric acid in the example 1 is changed to 2mL, 3mL, 5mL, 7mL respectively by 4mL, other conditions and steps are constant, Rb 4 CdCl 6 : The fluorescence efficiencies of Sn are 90.1%, 91.4%, 94.6%, and 90.8%, respectively, so 4mL of hydrochloric acid is the most optimal amount.
Embodiment 3
[0025] In Example 1, since Sn 2+ Instability, it is very easy to generate oxidation reaction to form Sn in high temperature acidic solution 4+ , has an adverse effect on the fluorescence quantum yield of the product, so hypophosphorous acid must be added. The amount of hypophosphorous acid was changed from 200 μL in Example 1 to 50 μL, 150 μL, and 300 μL respectively, and other conditions and steps remained unchanged. The measured fluorescence efficiencies of each product were 49.1%, 89.8%, and 80.3%, respectively, so the amount of hypophosphorous acid was 200 μL best.
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence quantum yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com