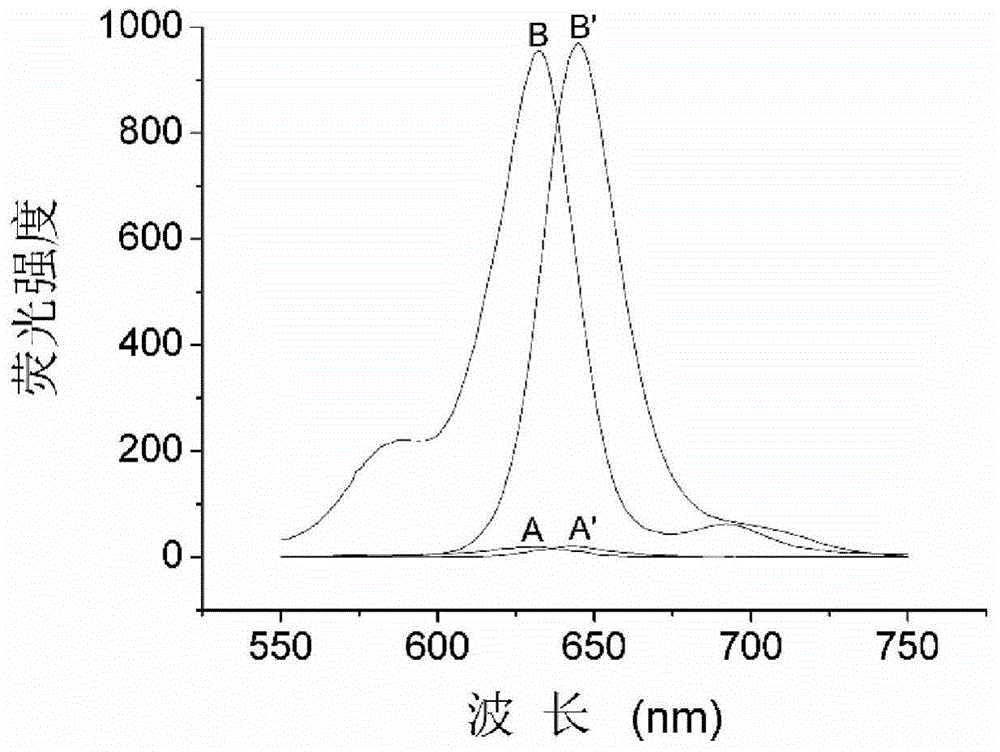
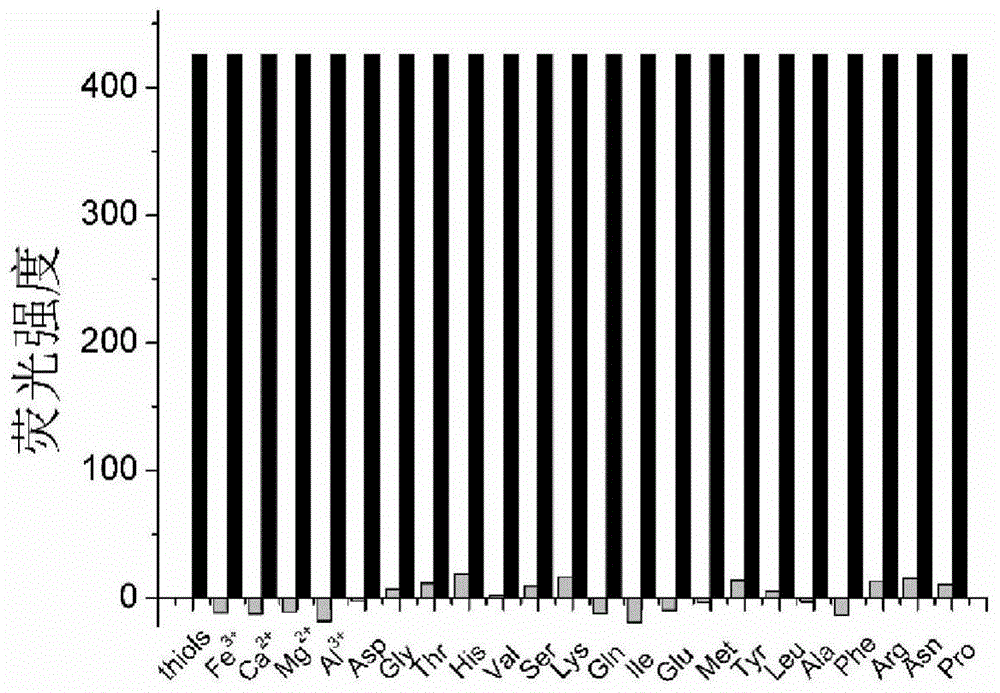
Long-wavelength fluorescence probe, and preparation method and application thereof
A fluorescent probe, long-wavelength technology, applied in the field of fluorescent probes, can solve problems such as the influence of fluorescence intensity, and achieve the effects of less biological damage, simple preparation method and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
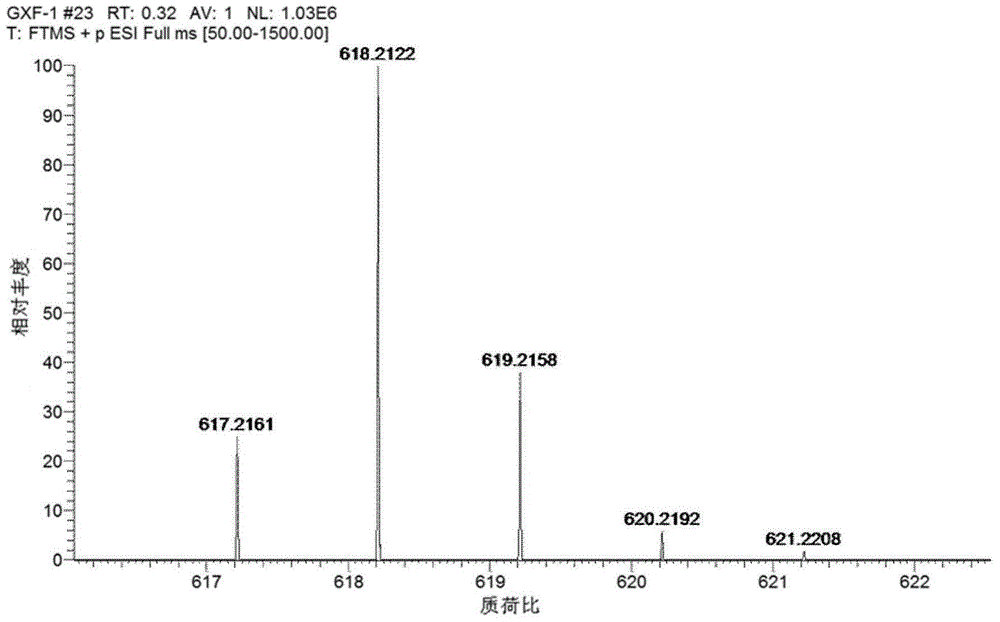
[0034] Dissolve 1,3,5,7-tetramethyl-8-phenyl-(2'-nitro)boron difluoride dimethylpyrrole (0.65 mmol) in benzene (15 mL), then add benzaldehyde in sequence (1.30mmol), piperidine (0.52mL) and glacial acetic acid (0.39mL), refluxed at 75°C for 30h with an oil-water separator, distilled off the solvent under reduced pressure, and purified by column chromatography with dichloromethane as eluent to obtain 1,7-Dimethyl-3,5-distyryl-8-phenyl-(2'-nitro)-borondifluoride dipyrromethane (276.5 mg, 78% yield), the intermediate Product 1: Dissolve intermediate product 1 (276.5mg, 0.50mmol) in 47mL of glacial acetic acid, add 6.5g of zinc powder, stir at room temperature for 80min, filter with suction to obtain the filtrate, distill off the solvent under reduced pressure, rinse with dichloromethane The solution was purified by column chromatography to obtain 1,7-dimethyl-3,5-distyryl-8-phenyl-(2'-amino)-boron difluoride dipyrromethane (194.3 mg, yield 75%), namely the intermediate product 2...
Embodiment 2
[0036]Dissolve 1,3,5,7-tetramethyl-8-phenyl-(2'-nitro)boron difluoride dimethylpyrrole (1.56mmol) in 20mL of benzene, then add benzaldehyde (4.68 mmol), piperidine (1.3mL) and glacial acetic acid (1.1mL), heated to reflux at 80°C for 24 hours with an oil-water separator device, distilled off the solvent under reduced pressure, and purified by column chromatography with dichloromethane as eluent to obtain Blue solid powder compound 2,1,7-dimethyl-3,5-distyryl-8-phenyl-(2'-nitro)-borondifluoride dipyrromethane (680.4 mg, yield 80%), namely intermediate product 1; intermediate product 1 (680.4 mg, 1.24 mmol) was dissolved in 100 mL of glacial acetic acid, zinc powder (17.5 g) was added, stirred at room temperature for 90 min, and the filtrate was obtained by suction filtration, which was distilled off under reduced pressure Solvent, purified by column chromatography with dichloromethane as eluent to obtain blue solid powder compound 1,7-dimethyl-3,5-distyryl-8-phenyl-(2'-amino) ...
Embodiment 3
[0038] Dissolve 1,3,5,7-tetramethyl-8-phenyl-(2'-nitro)boron difluoride dimethylpyrrole (1.56mmol) in 20mL of benzene, then add benzaldehyde (6.25 mmol), piperidine (1.4mL) and glacial acetic acid (1.25mL), heated to reflux at 85°C for 28 hours with an oil-water separator, distilled off the solvent under reduced pressure, and purified by column chromatography with dichloromethane as eluent to obtain Blue solid powder compound 2,1,7-dimethyl-3,5-distyryl-8-phenyl-(2'-nitro)-borondifluoride dipyrromethane (671.9 mg, yield 79%), that is, intermediate product 1; intermediate product 1 (671.9 mg, 1.22 mmol) was dissolved in 104 mL of glacial acetic acid, zinc powder (18.3 g) was added, stirred at room temperature for 84 min, and the filtrate was obtained by suction filtration, which was distilled off under reduced pressure Solvent, using dichloromethane as the eluent for column chromatography purification, to obtain blue solid powder compound 3,1,7-dimethyl-3,5-distyryl-8-phenyl-(2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com