Preparation method and application of anhydrous peroxycarboxylic acid
An anhydrous peroxycarboxylic acid and carboxylic acid technology, applied in electrolysis process, organic chemistry, electrolysis components, etc., can solve the problem of unsuitability for ε-caprolactone, etc., and achieve high reaction yield, mild reaction conditions, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
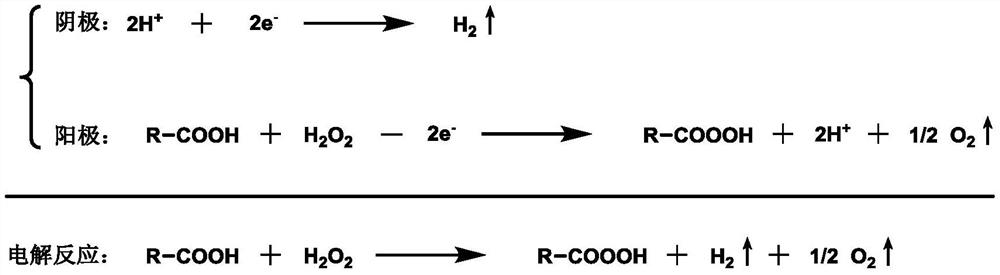
Image
Examples
Embodiment 1
[0036] Weigh 500g of methyl butyrate and 25g of 10wt% hydrogen peroxide and add them to the flask. A water separator is installed on the flask, and a condenser is installed on the water separator. Magnetic stirring, control the pressure 5kPa, heat the flask to raise the temperature, the gas phase flows through the water separator after being condensed by the condenser tube, the water is separated through the water separator, and the rest of the condensate flows back into the flask. When the temperature in the flask gradually increased to 28.6°C and remained constant, the heating was stopped, and the vacuum was broken after the system temperature returned to room temperature.
[0037] Transfer all the materials in the above flask to the electrolytic cell, weigh 0.50 g of cobalt acetate, 0.50 g of platinum acetate and 6.48 g of butyric acid into the electrolytic cell, and start stirring to mix the materials in the electrolytic cell evenly. Then, the anode (platinum electrode, 2....
Embodiment 2
[0039] Weigh 500g of ethyl propionate and 166.67g of 50wt% hydrogen peroxide and add them to the flask. A water separator is installed on the flask, and a condenser is installed on the water separator. Magnetic stirring is used to control the pressure to 30kPa, and the flask is heated to raise the temperature. The gas phase is condensed by the condenser tube and then flows through the water separator. The water is separated through the water separator, and the rest of the condensate is returned to the flask. When the temperature in the flask gradually increased to 65.2°C and remained constant, the heating was stopped, and the vacuum was broken after the system temperature returned to room temperature.
[0040] Transfer all the materials in the above flask to the electrolytic cell, weigh 2.50g of silver acetate, 0.50g of ruthenium acetate and 363.03g of propionic acid and add them to the electrolytic cell, start stirring to mix the materials in the electrolytic cell evenly. The...
Embodiment 3
[0042] Weigh 500g of propyl acetate and 41.67g of 30wt% hydrogen peroxide and add them to the flask. A water separator is installed on the flask, and a condenser is installed on the water separator. Magnetic stirring is used to control the pressure to 18kPa, and the flask is heated to raise the temperature. The gas phase is condensed by the condenser tube and then flows through the water separator. The water is separated through the water separator, and the rest of the condensate flows back into the flask. When the temperature in the flask gradually increased to 54.5°C and remained constant, the heating was stopped, and the vacuum was broken after the system temperature returned to room temperature.
[0043]Transfer all the materials in the above-mentioned flask to the electrolytic cell, weigh 0.83g of nickel acetate, 0.50g of iridium acetate and 33.11g of acetic acid into the electrolytic cell, start stirring to mix the materials in the electrolytic cell evenly. Then, the ano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com