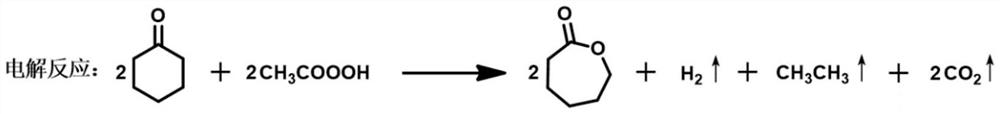
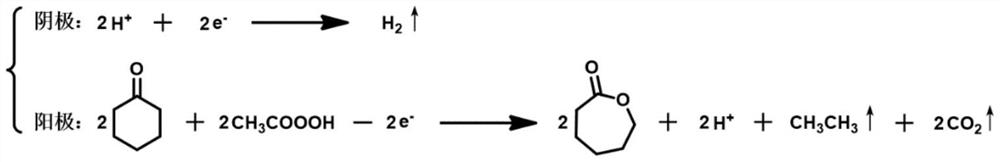
Method for preparing epsilon-caprolactone from cyclohexanone
A technology of cyclohexanone and caprolactone, applied in the field of electrochemical preparation of ε-caprolactone, to achieve enhanced oxidation reaction effect, simple process flow and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0044] The initial peracetic acid solution of the present invention is prepared with reference to the method of patent document CN106349140B, and its specific preparation process is:
[0045] 200 g of bissulfonic acid-based polystyrene resin was charged into a reactor with a volume of 4 L, and 1200 g of acetic acid and 880 g of ethyl acetate were added into the reactor.
[0046] The reactor has a packed distillation column and a reflux condenser with a settling tank. Stir this solution at about 20kPa (absolute pressure), heat it to about 40° C. with an oil bath, and add a total amount of 440 g of 50 wt % hydrogen peroxide solution into the reactor. Control the temperature of the reactor at about 40-45°C, introduce the organic phase in the heterogeneous azeotrope condensed by the low-temperature system in the reflux condenser into the adsorption tower, which is equipped with molecular sieves, and the organic phase passes through the molecular sieves Adsorption dehydration, the...
Embodiment 1
[0049] Weigh 224.1g of the initial peracetic acid solution and 375.9g of ethyl acetate into the flask, mix evenly and prepare an 8wt% peracetic acid solution.
[0050] Transfer all the above peracetic acid solution to the electrolytic cell, weigh 0.50 g of lead tetraacetate and 2.50 g of lithium chloride and add them to the electrolytic cell, start stirring to mix the materials in the electrolytic cell evenly. Then, the anode (lead electrode, 2.5×6cm 2 ) and cathode (lead electrode, 2.5×6cm 2 ) into the reaction system, turn on magnetic stirring and energize, and keep the current constant at 4.8A (current density 3200A / m 2 ), control the temperature at 35°C, add cyclohexanone dropwise to the electrolytic cell by an advection pump, carry out the electrolysis reaction, and control the reaction time of dropwise adding cyclohexanone (74.3g) at 3h, continue after the dropwise addition of cyclohexanone solution ends Insulation reaction 3h. Collect 675.7 g of the reaction solution...
Embodiment 2
[0052] Weigh 140.1g of the initial peracetic acid solution and 9.9g of ethyl acetate into the flask, mix well and prepare a 20wt% peracetic acid solution.
[0053] Transfer all the above peracetic acid solution to the electrolytic cell, weigh 0.75g of copper acetate and 0.75g of cobalt chloride and add them to the electrolytic cell, start stirring to mix the materials in the electrolytic cell evenly. Then, the anode (platinum electrode, 2.5×6cm 2 ) and cathode (platinum electrode, 2.5×6cm 2 ) into the reaction system, turn on magnetic stirring and energize, and keep the current constant at 18A (current density 12000A / m 2 ), control the temperature at 65°C, drop cyclohexanone in the electrolytic cell by an advection pump, carry out the electrolysis reaction, and drop the reaction time of cyclohexanone solution (185.8g cyclohexanone and 433.6g butyl butyrate) at 1h, after the dropwise addition of the cyclohexanone solution was completed, the insulation reaction was continued f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com