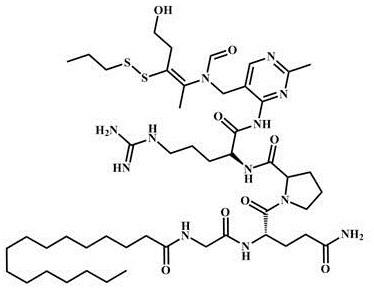
Peptide conjugated derivatives based on palmitoyl tetrapeptide-7, preparation methods and uses
A technology of palmitoyl tetrapeptide and derivatives, which is applied in the fields of preparation methods of peptides, chemical instruments and methods, preparations for skin care, etc., can solve the problems of few or few derivatization or conjugation, and achieve excellent transdermal absorption. Activity, increased efficacy, high anti-inflammatory activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
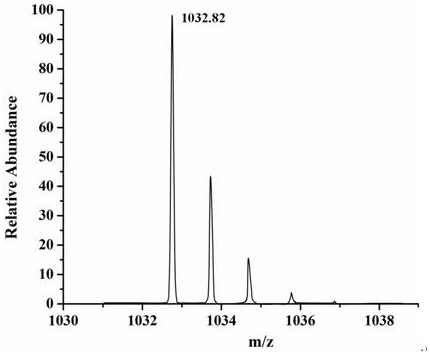
[0036] Preparation of peptide-conjugated derivatives based on palmitoyl tetrapeptide-7:
[0037]Dissolve palmitoyl tetrapeptide-7 in methanol solution (the ratio of solid to liquid is 1 g: 6 mL), and add DCC (the molar ratio of palmitoyl tetrapeptide-7 is 1.2: 1) to it, and react at room temperature for 2.5 h, then add prosuthiamine (the molar ratio to palmitoyl tetrapeptide-7 is 1.25:1) and triethylamine (the molar ratio to palmitoyl tetrapeptide-7 is 2.2:1), and stir the reaction at room temperature for 18 h; then distill off the solvent under reduced pressure, add saturated sodium bicarbonate (equal volume with methanol / triethylamine mixed solution) solution, then wash it twice with diethyl ether (equal volume with methanol / triethylamine mixed solution), The aqueous phase was adjusted to pH 2.5 by adding 1 M sodium bisulfate aqueous solution, extracted three times with ethyl acetate (2 times the volume of methanol / triethylamine mixed solution), and the organic phase was seq...
Embodiment 2
[0041] Preparation of Alcohol Gels Based on Peptide Conjugated Derivatives of Palmitoyl Tetrapeptide-7:
[0042] Mix the peptide-conjugated derivatives based on palmitoyl tetrapeptide-7 with alcohol, stir, heat up and dissolve, place it at 45°C, and let it age for 6.5 hours to obtain the alcohol gel; among them, the peptides based on palmitoyl tetrapeptide-7 The concentration of the conjugated derivative was 6.2 mg / mL.
[0043] The alcohol used in this example is a mixture of ethanol and sorbitol, with a volume ratio of 2.5:1.
Embodiment 3
[0045] The difference between the preparation of the palmitoyl tetrapeptide-7-based peptide-conjugated derivative alcohol gel and Example 2 is that the concentration of the palmitoyl tetrapeptide-7-based peptide-conjugated derivative is 3.6 mg / mL; the alcohol used is A mixture of propanol and panthenol in a volume ratio of 3:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com