Preparation method of deuterated aromatic compound
A technology for aromatic compounds and compounds, applied in the field of preparation of deuterated aromatic compounds, can solve the problems of low deuterated rate, low product yield, poor selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a kind of preparation method of deuterated aromatic compound, comprises the following steps:
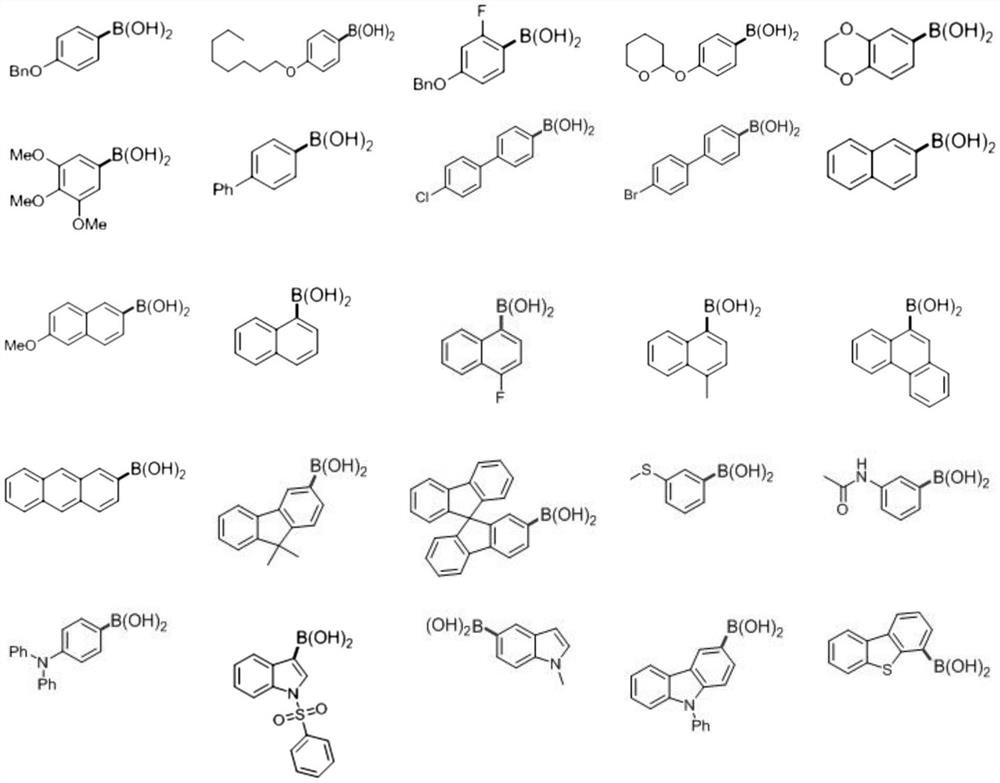
[0030] Mix aryl boronic acid, photocatalyst, Lewis base catalyst, mercaptan catalyst, deuterated water and organic solvent, and perform deboronated deuterated reaction under visible light irradiation condition to obtain deuterated aromatic compound.
[0031] In the present invention, the photocatalyst is preferably Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 , the specific structure is as follows:
[0032]
[0033] In the present invention, the Lewis base catalyst preferably includes one or more of quinuclidine, quinuclidin-3-ol, triethylamine, triphenylphosphine and 4-dimethylaminopyridine; the sulfur Alcohol catalysts preferably include substituted or unsubstituted thiophenols, C 4 ~C 16 One or more of the alkylthiols, the substituted thiophenol is preferably alkylthiophenol, more preferably p-methylthiophenol, p-tert-butylthiophenol or p-isopropyl Thioph...
Embodiment 1
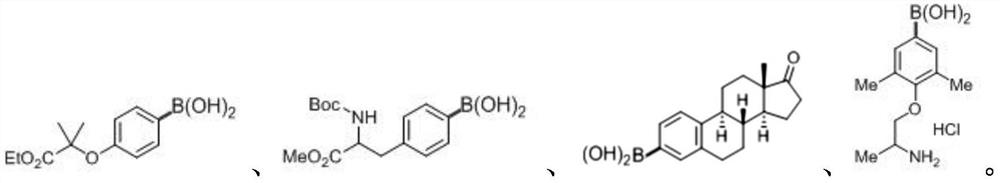
[0061] Embodiment 1: Synthesis of 2-methyl-2-(phenoxy-4-d) ethyl propionate:
[0062] Weigh 0.2mmol (4-((1-ethoxy-2-methyl-1-oxopropan-2-yl) oxy)phenyl) boronic acid, 0.004mmol photocatalyst Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 , 0.04mmol quinuclidin-3-ol, 0.08mmol tert-butyl mercaptan in an 8mL reaction bottle, then add 1mL deuterated chloroform, 1mL deuterated water, blow argon gas into the reaction bottle for 30s, and use 470nm blue light at 36W React under irradiation for 24 hours, spin off the solvent, and the resulting crude product was subjected to column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain a white solid with a yield of 85% and a melting point of 124-125°C. 1 HNMR (400MHz, CDCl 3 )δ7.25(d, J=8.4Hz, 2H), 6.87(d, J=8.4Hz, 2H), 4.26(q, J=7.2Hz, 2H), 1.62(s, 6H), 1.26(t, J=7.2Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ174.4, 155.5, 129.1, 121.8 (t, J=24.5Hz), 119.1, 79.0, 61.4, 25.4, 14.1. HRMS (EI-FTMS) m / z: [M] + ·Calcd for C 12 h 15 do 3 209.116...
Embodiment 2
[0063] Embodiment 2: the synthesis of tyrosine-4-d:
[0064] Weigh 0.2mmol (4-(2-((tert-butoxycarbonyl)amino)-3-methoxy-3-oxopropyl)phenyl)boronic acid, 0.004mmol photocatalyst Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 , 0.04mmol quinuclidin-3-ol, 0.08mmol tert-butyl mercaptan in 8mL reaction bottle, then add 1mL deuterated chloroform solution, 1mL deuterated water, blow argon gas into the reaction bottle for 30s, at 36W at 470nm The reaction was performed under blue light irradiation for 24 hours, and the solvent was spun off. The obtained crude product was subjected to column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain a white solid with a yield of 62% and a melting point of 187-188°C. 1 HNMR (400MHz, CDCl 3 )δ7.28(d, J=7.2Hz, 2H), 7.12(d, J=7.2Hz, 2H), 5.00(d, J=7.2Hz, 1H), 4.59(dd, J=13.6, 6.0Hz, 1H), 3.71(s, 3H), 3.08(qd, J=13.6, 6.0Hz, 2H), 1.42(s, 9H). 13 C NMR (100MHz, CDCl 3 )δ172.4, 155.1, 136.0, 129.3, 128.6, 128.5, 127.8 (t, J = 23.5Hz), 127.1, 80....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com