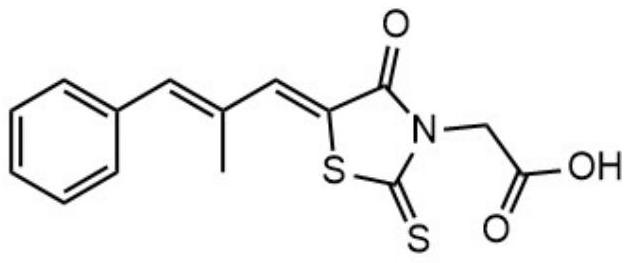
Application of epalrestat or pharmaceutically acceptable salt thereof in preparation of medicine for preventing and/or treating bacterial infection diseases
A technology for epalrestat and bacterial infection, applied in the field of biomedicine, which can solve the problems of few types of metallo-β-lactamase inhibitors, few optional drugs for infection treatment, and low safety, so as to overcome bacterial resistance , Improve anti-infection effect, increase sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
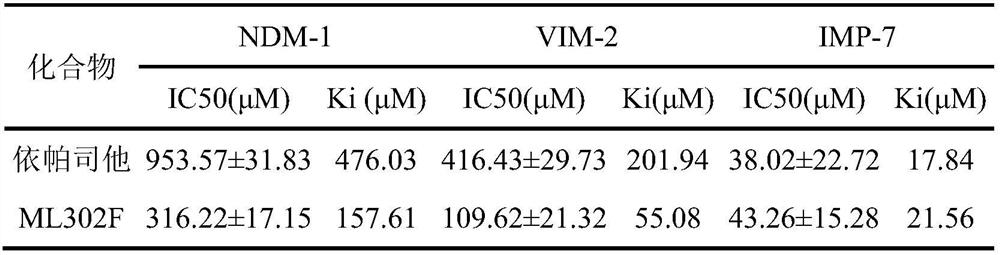
[0030] Example 1 Detection of epalrestat on the inhibitory activity of IMP-7 metallo-β-lactamases
[0031] test group:
[0032] S1. Firstly mix metallo-β-lactamases (IMP-7, VIM-2, NDM-1 with final concentrations of 2nM, 4nM, 10nM) and different concentrations of epalrestat in buffer in a 96-well plate , incubate at 25°C for 30 minutes to fully combine the inhibitor with the enzyme;
[0033] S2. Add meropenem dissolved in the buffer solution (the final test concentration of IMP-7 and VIM-2 is 50 μM, and the final test concentration of NDM-1 is 100 μM) into a 96-well plate, and measure the change of the absorbance of the system immediately after mixing evenly. Record data.
[0034] Since the absorbance of the substrate meropenem decreases after being hydrolyzed by the enzyme, the degree of hydrolysis of the substrate can be characterized by measuring the change of the absorbance of meropenem. Calculate the residual activity of the enzyme after different concentrations of epal...
Embodiment 2
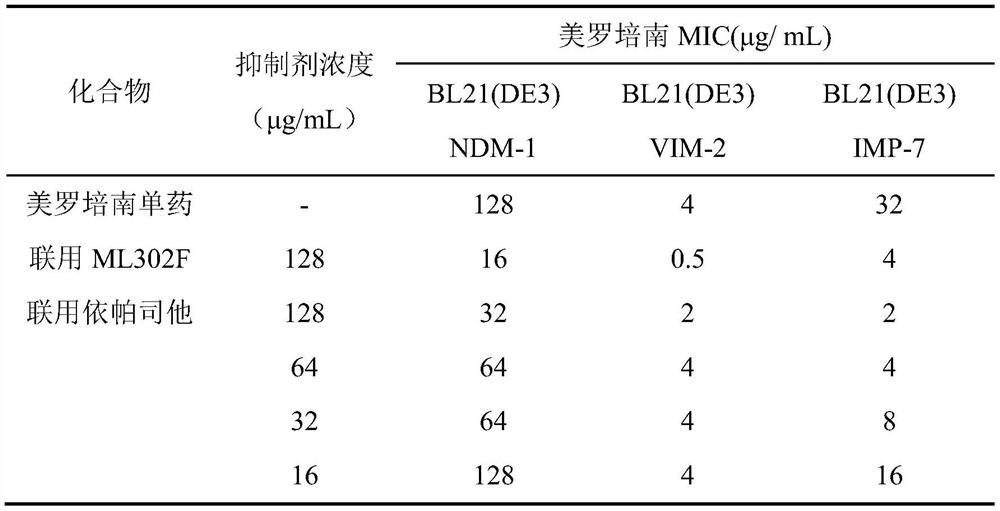
[0038] Example 2 In vitro antibacterial detection of metallo-β-lactamase inhibitors combined with meropenem
[0039] S1. Inoculum Preparation
[0040] Pick colonies from the fresh culture (Escherichia coli BL21 (DE3) expressing metalloβ-lactamases) cultured on the plate for 18-24 hours, inoculate them into LB liquid medium of 3 mL of kanamycin, and culture After 4 to 6 hours, adjust the colony concentration to 0.5 McFarland turbidimetric standard bacterial suspension, and dilute the above bacterial suspension 1:100 with LB broth before use. During the dilution process, 20mM isopropyl-β-D-thiogalactopyranoside (IPTG) was added at a ratio of 1:20, so that the diluted bacterial solution contained 1mM IPTG.
[0041] S2. Preparation of epalrestat solution and bacterial inoculation
[0042] ① Dilution of meropenem: Prepare 512 μg / mL meropenem solution as the working solution, add 100 μL of the working solution to the first column of the 96-well plate (wells A1-H1), add 50 μL of me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com