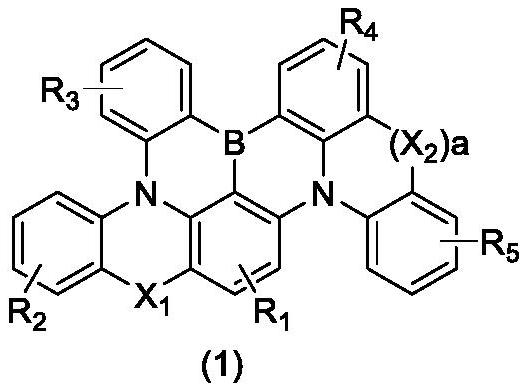
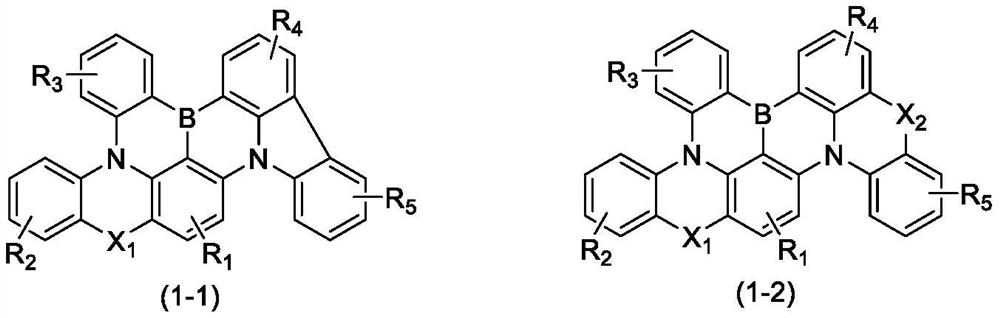
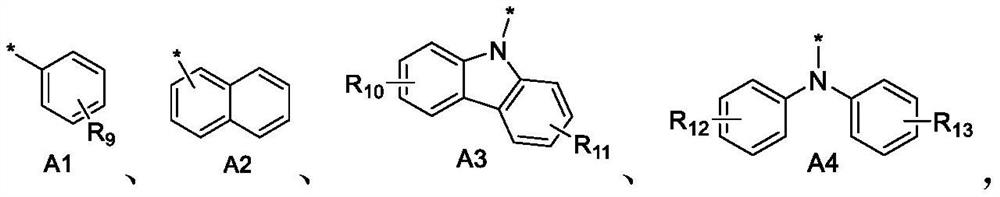
Boron-based compound and application thereof in organic electroluminescent device
A technology of boron-based compounds and compound structures, applied to boron-based compounds and their application in organic electroluminescent devices, can solve the problems of device efficiency roll-off, difficult and unstable processes of carrier transfer and energy transfer, etc. , to avoid annihilation or quenching, weaken non-radiative transitions, and improve thermodynamic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] It should be understood that the specific embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
[0028] Synthesis Example 1: Synthesis of Compound (2)
[0029] In the reactor, put 10-([1,1'-biphenyl]-4-yl)-2-chloro-7-phenyl-10hydrogen-phenoxazine (20mmol, 8.92g), 9hydrogen- Carbazole (20mmol, 3.34g), potassium carbonate (40mmol, 5.76g) and toluene 100mL, blow nitrogen, add cuprous iodide 0.38g (2mmol) and o-phenanthroline 0.72g (4mmol), heat and reflux and stir for 8h . The temperature was cooled to room temperature, filtered, the liquid phase was distilled under reduced pressure, mixed with the filter cake, and purified by silica gel column chromatography to obtain 8.65 g of the compound represented by the following chemical formula (2a), with a yield of 75%;
[0030]
[0031] In the reactor, the compound represented by the above (2a) (10mmol, 5.77g) was dissolved in 50mL of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com