Kit for evaluating coronary artery disease and application thereof
A coronary artery disease and kit technology, applied in the field of kits, can solve the problems of lack of selectivity, low precision and accuracy, low throughput, etc., and achieve good sensitivity and specificity, high precision and accuracy, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Subject sample collection, preservation and transportation
[0110] Reagents and test supplies for collecting subjects' samples can be included in the kit of the present invention, such as the EDTA anticoagulant tube (purple cap) used below.
[0111] (1) Sample collection
[0112] Collect 3ml of the subject's blood through venous blood collection, and separate the plasma by centrifugation (centrifugation conditions: 1300g, 10min) within 6 hours after blood collection; preferably, EDTA anticoagulant tubes (purple cap) are the first choice to separate plasma.
[0113] (2)Sample preservation
[0114] If it cannot be used for detection immediately, the separated plasma must be stored at 2-8°C or -20°C and protected from light.
[0115] (3) Sample transportation
[0116] Plasma was shipped at 4°C on blue ice.
Embodiment 2
[0117] Example 2: Pretreatment of the plasma sample collected in Example 1 before LC-MS / MS detection (protein rapid precipitation method)
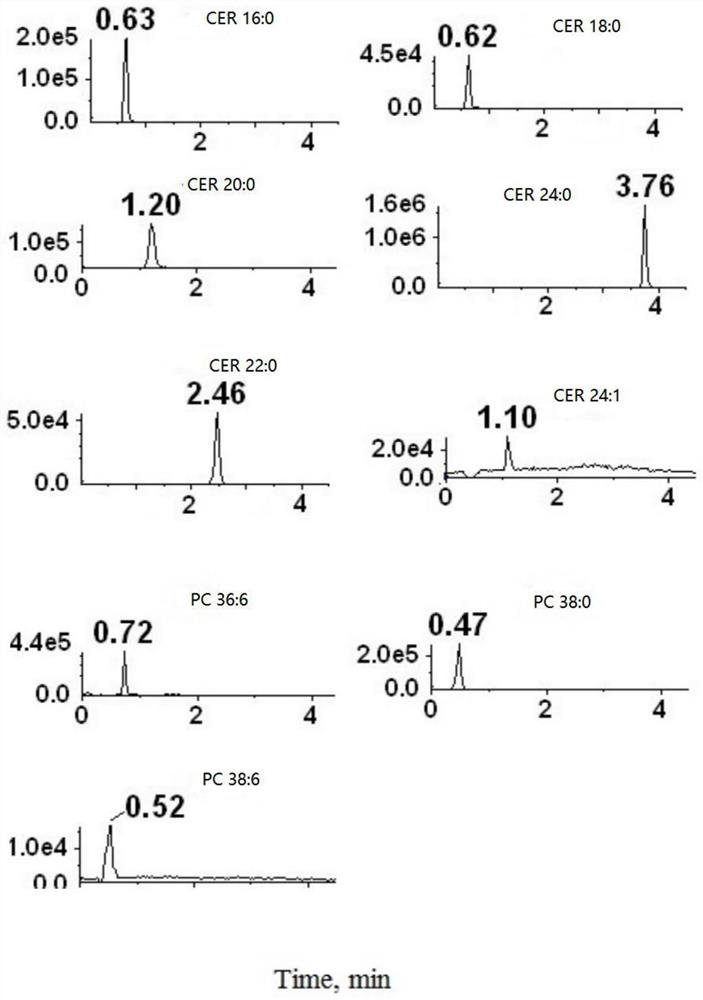
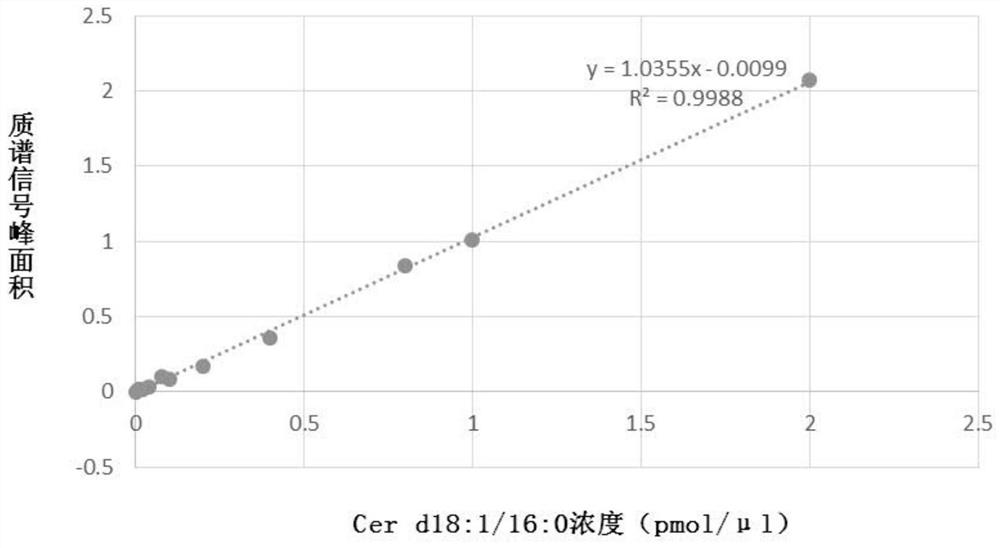
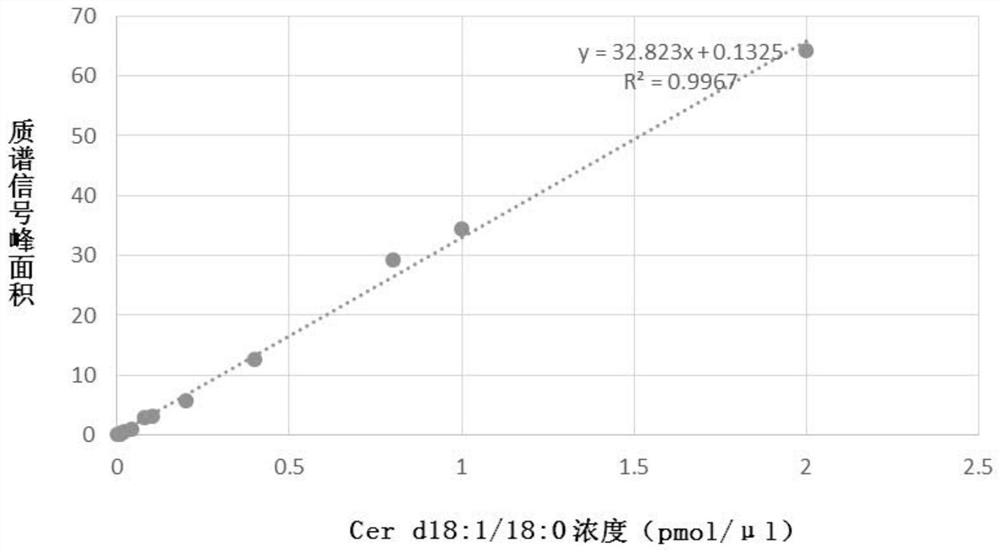
[0118] Reagents and test supplies for pretreatment of plasma samples can be included in the kit of the present invention, such as: protein precipitation (PPT) solvent: ethyl acetate: isopropanol (2:8, v / v), this The kit of the invention may also include: for making Cer(d18:1 / 16:0), Cer(d18:1 / 18:0), Cer(d18:1 / 20:0), Cer(d18:1 / 22:0), Cer(d18:1 / 24:0), Cer(d18:1 / 24:1) and PC aa C36:6, PC aa C38:0, PC aa C38:6) standard curve of phospholipids The pure product of the compound (commercially available product) or the pure product storage solution prepared by methanol solvent (the concentration of the storage solution can be 500pmol / μl); the four ceramide internal standards (IS, that is, the four ceramides corresponding to the four ceramides) prepared by methanol solvent Deuterium) solution, its concentration is D 7 -Cer d18:1 / 16:0: 0.125 pmol / μ...
Embodiment 3
[0120] Example 3: Optimization of LC-MS / MS detection and analysis conditions.
[0121] The optimized LC-MS / MS detection and analysis conditions and equipment used in this example can be described in the instructions of the kit of this application. Or / and the reagents needed to optimize the detection and analysis conditions of LC-MS / MS are used as a part of the reagents in the kit of the present invention.
[0122] LC-MS / MS analysis was performed on a Thermo SCIENTIFIC TSQ Quantitative mass spectrometer coupled with a Thermo SCIENTIFIC UPLC Ultimate3000. Electrospray ionization (ESI) in positive ion mode employs multiple reaction monitoring (MRM). Instrument control and data acquisition were controlled using the Thermo SCIENTIFIC TSQ Quantitative companion software.
[0123] Before the detection work on the subject's plasma samples, the detection conditions were comprehensively optimized to obtain D 7 -Cer d18:1 / 16:0, D 7 -Cer d18:1 / 16:0, D 7 -Cer d18:1 / 18:0, D 7 -Cer d18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com