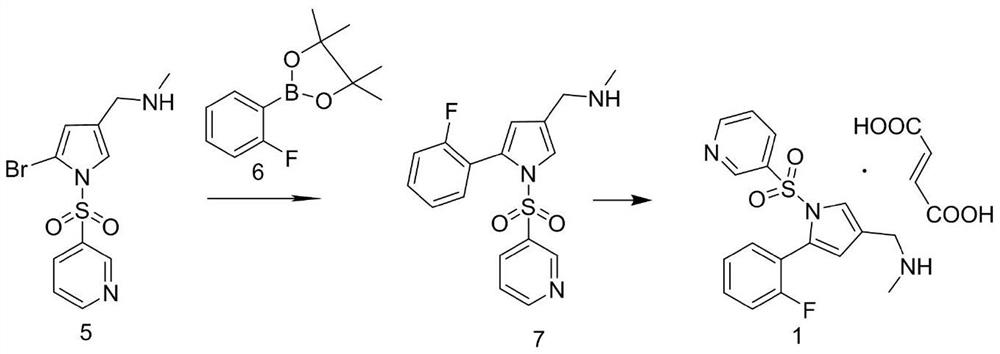
Vonoprazan intermediate as well as preparation method and application thereof
An intermediate and reaction technology, applied in the field of medicinal chemistry, can solve the problems of residual toxic impurities and cumbersome operation process, and achieve the effects of high reaction efficiency, avoiding hydrogenation reaction, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
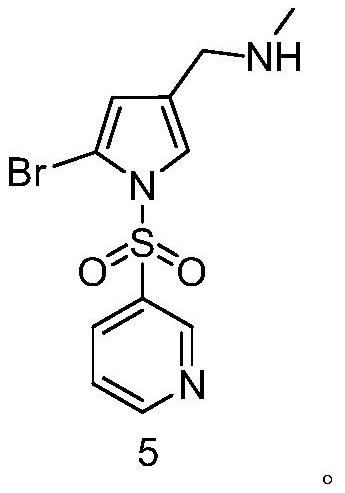
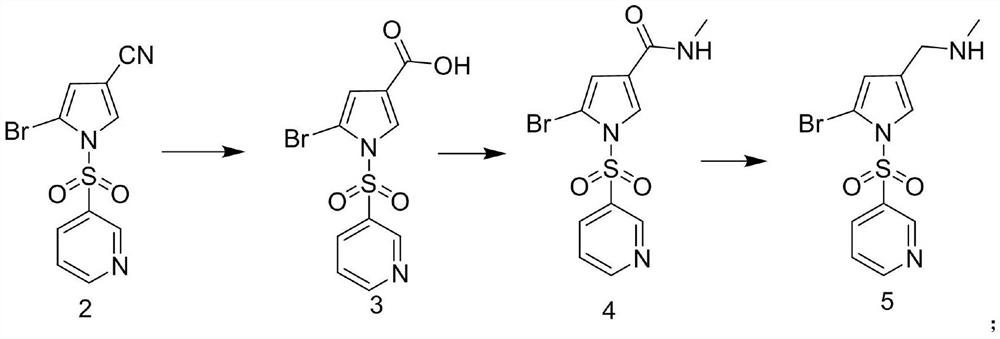
[0073] Preparation of Example 1 Compound 5
[0074] Dissolve 31.2g of 1-(3-pyridylsulfonyl)-2-bromo-1H-pyrrole-3-carbonitrile in 320mL of ethanol aqueous solution (80%), add 5.4mL of concentrated sulfuric acid solution, heat up to 45°C, and keep the reaction for 8h After the reaction, 500 mL of purified water was added, extracted three times with 500 mL of ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and the filtrate was dried under reduced pressure to obtain an oily compound 3, namely 1-(3-pyridylsulfonyl)- 2-Bromo-1H-pyrrole-3-carboxylic acid, 90.1% yield, ESI-MS (m / z): 332.25 [M+H] + .
[0075] Dissolve 29.82 g of oily compound 3 in 170 mL of acetonitrile solution, then add 6.08 g of solid methylamine hydrochloride, add 9.7 mL of diethylamine, add 0.1 mL of DMF, stir to cool down to 5 °C, add 9 mL of oxalyl chloride solution dropwise, add dropwise After the completion, the temperature was raised to 40 °C and stirred for 2 ...
Embodiment 2
[0079] Preparation of Example 2 Compound 5
[0080] Dissolve 31.2g of 1-(3-pyridylsulfonyl)-2-bromo-1H-pyrrole-3-carbonitrile in 460mL of aqueous ethanol solution (60%), add 10mL of concentrated sulfuric acid solution, heat up to 45°C, and keep the reaction for 8h, After the reaction, 500 mL of purified water was added, extracted three times with 500 mL of ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and the filtrate was dried under reduced pressure to obtain an oily compound 3, namely 1-(3-pyridylsulfonyl)-2 -Bromo-1H-pyrrole-3-carboxylic acid, yield 87.1%, ESI-MS (m / z): 332.25 [M+H] + .
[0081] Dissolve 28.92 g of oily compound 3 in 150 mL of acetonitrile solution, then add 6.48 g of solid methylamine hydrochloride, add 9.1 mL of diethylamine, add 0.2 mL of DMF, stir to cool down to 5 °C, add 7.5 mL of oxalyl chloride solution dropwise, dropwise After the addition, the temperature was raised to 40 °C and stirred for 2 h. A...
Embodiment 3
[0083] Preparation of Example 3 Compound 5
[0084] 31.2g of 1-(3-pyridylsulfonyl)-2-bromo-1H-pyrrole-3-carbonitrile was dissolved in 160mL of ethanol aqueous solution (90%), 8mL of concentrated sulfuric acid solution was added, the temperature was raised to 60°C, and the reaction was incubated for 7h. After the reaction, 500 mL of purified water was added, extracted three times with 500 mL of ethyl acetate, the organic phase was collected, dried over anhydrous sodium sulfate, filtered, and the filtrate was dried under reduced pressure to obtain an oily compound 3, namely 1-(3-pyridylsulfonyl)-2 -Bromo-1H-pyrrole-3-carboxylic acid, 87% yield, ESI-MS (m / z): 332.26 [M+H] + .
[0085] Dissolve 28.89 g of oily compound 3 in 290 mL of acetonitrile solution, then add 6.15 g of solid methylamine hydrochloride, add 11 mL of diethylamine, add 0.1 mL of DMF, stir to cool down to 5 °C, add 11 mL of oxalyl chloride solution dropwise, and complete the dropwise addition Then the temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com