Method for determining urokinase activity by chromophoric substrate method
A chromogenic substrate, urokinase technology, applied in color/spectral characteristic measurement, measuring device, material analysis through optical means, etc., can solve the problem that the sample cannot be fully reacted, cannot meet the production detection requirements, and affects the optimal enzyme Respond to problems such as pH, achieve objective test results, meet production testing needs, and have a wide range of urokinase detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 The establishment of linear relationship regression equation
[0043] The present embodiment provides the method for the establishment of linear relationship regression equation, and concrete steps are as follows:
[0044] (1) Prepare gelatin-Tris buffer solution (pH8.8):
[0045] Tris 6.06g and sodium chloride 2.22g are mixed, add 700mL purified water to dissolve, obtain mixed liquid A;
[0046] Separately get 10g of gelatin and add an appropriate amount of purified water for heating and dissolving, cool the solution to room temperature and mix it with mixed solution A, shake it up to obtain mixed solution B;
[0047] Adjust the pH value of the mixture B to 8.8 with dilute hydrochloric acid, and then add purified water to dilute to 1000 mL.
[0048] (2) Preparation of chromogenic substrate solution:
[0049] Prepare the chromogenic substrate solution that concentration is 0.5mmol / L with gelatin-Tris buffer solution (pH8.8), add in the enzyme standard we...
Embodiment 2
[0058] This embodiment provides a method for determining the activity of urokinase by a substrate chromogenic method, specifically as follows:
[0059] Selecting the batch number produced by Beijing Saisheng Pharmaceutical Co., Ltd. is 20091223 urokinase preparation as the sample to be tested, and the marked amount is 1139 units;
[0060] Quantitatively dilute with gelatin-Tris buffer to make a solution with a concentration of 20-100U / mL, add it to the enzyme-labeled well plate with substrate, and set 3 duplicate holes;
[0061] Put the above enzyme-labeled well plate into a microplate reader at 37°C for 23 minutes;
[0062] The absorbance of the enzymatic hydrolysis product p-nitroaniline (PNA) in each well at the wavelength of 405nm is 0.4988;
[0063] Substitute this value into the standard curve obtained in Example 1, and the calculated urokinase activity in this preparation is 1080.83 units.
[0064] The results show that the activity value obtained by the standard curv...
Embodiment 3
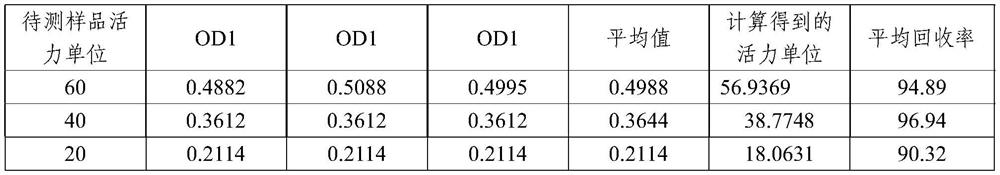
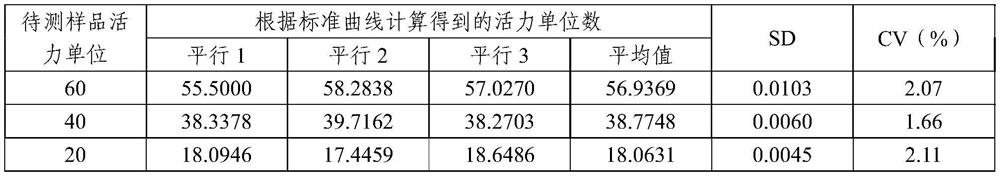
[0065] Embodiment 3 average rate of recovery
[0066] According to the solution preparation method in Example 1, 200 μl of chromogenic substrate S-2444 solution was added to the reaction wells of the microtiter plate, and a standard curve was prepared with urokinase as a standard, and 3 duplicate holes were set for each activity unit point of the curve , select the batch number 20091223 preparation produced by Beijing Saisheng Pharmaceutical Co., Ltd. as the test product (enzyme activity label value is 1139 units), select 60 units, 40 units and 20 units as high, medium and low concentrations respectively, each sample 3 duplicate holes.
[0067] Immediately put the above-prepared microplate plate into a microplate reader preheated to 37°C for 20 minutes, and then measure the absorbance value A405 of the enzymatic hydrolysis product p-nitroaniline (PNA) in each well at a wavelength of 405nm to make the enzyme Active standard curve, measured to obtain the absorbance A405 value o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com