Method for synthesizing pseudouridine by chemical enzyme method
A chemical enzymatic method, pseudouridine technology, applied in the directions of biochemical equipment and methods, botanical equipment and methods, enzymes, etc., can solve the problems of long synthesis steps, low yield, complicated purification process, etc., and achieve short reaction time. , the effect of high conversion rate and simple purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Chemical Synthesis of Ribose-5-Phosphate
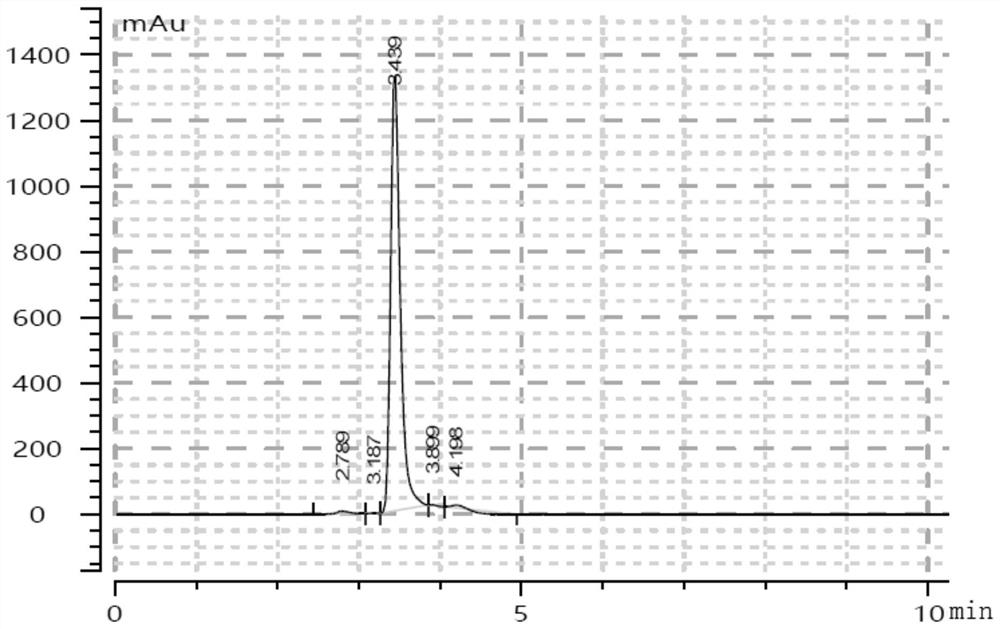
[0026] Dissolve 50g of AMP in 3L of 1M HCl, heat to 100°C, and stir for 1h. Then cool down to room temperature, adjust the pH of the system to 8.2 with a saturated KOH solution, add isopropanol to precipitate a precipitate, which is the crude ribose-5-phosphate. Add the crude ribose-5-phosphate solution to the ion exchange resin, use 0.2, 0.4, 0.6, 0.8 and 1.0M chloroacetic acid solution to elute, purify to obtain a purified ribose-5-phosphate solution, and freeze-dry to obtain 30g ribose-5- phosphoric acid. The ribose-5-phosphate was 85% pure as determined using HPLC equipped with an evaporative light scattering detector.
[0027] The detection method of ribose-5-phosphate is as follows:
[0028] Chromatographic column: Waters XBridge BEH Amide, 250×4.6mm, 5μm;
[0029] Column temperature: 40°C;
[0030] Flow rate: 1mL / min;
[0031] Mobile phase A: acetonitrile;
[0032] Mobile phase B: 2g / L ammonium acetat...
Embodiment 2
[0035] Expression, purification and mutation of embodiment 2 pseudouridine-5-phosphoglucosidase
[0036] 1) Expression and purification of pseudouridine-5-phosphoglucosidase
[0037] Pseudouridine-5-phosphoglucosidase is derived from Dorea formicigenerns ATCC 27755, which is codon-optimized pseudouridine-5-phosphoglucosidase for Escherichia coli, and its two ends use NdeI and XhoI restriction sites respectively. Use restriction endonucleases NdeI and XhoI to digest the target gene and pET-28a plasmid, recover the target gene fragment and vector fragment by agarose gel electrophoresis, use T4 DNA ligase to carry out the ligation reaction, and transform Escherichia coli DH5α competent , coated plate with kanamycin added. After picking a single clone for colony PCR verification, select the successfully connected strain to extract the plasmid for sequencing to verify the accuracy of the target fragment sequence.
[0038] After transforming the BL21(DE3) strain with the correctly...
Embodiment 3
[0043] Embodiment 3 synthetic pseudouridine monophosphate
[0044] 1) synthesis
[0045] Weigh 220.8g ribose-5-phosphate, 4.8g MnCl 2 4H 2 O, dissolve it using 5 L of deionized water. Weigh 53.7g of uracil again, and dissolve it with 4.8L of deionized water (heating to 80°C helps dissolve).
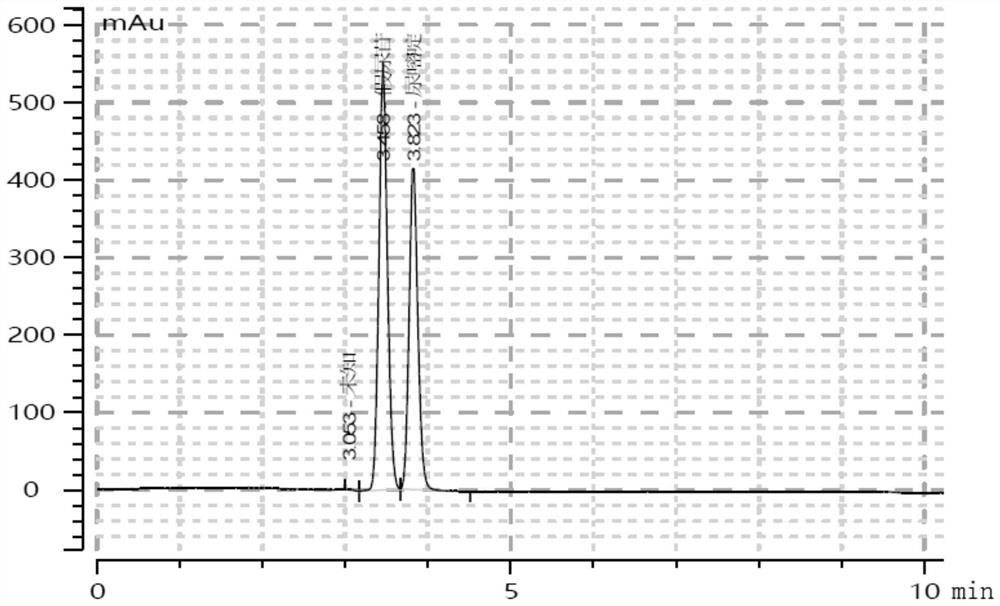
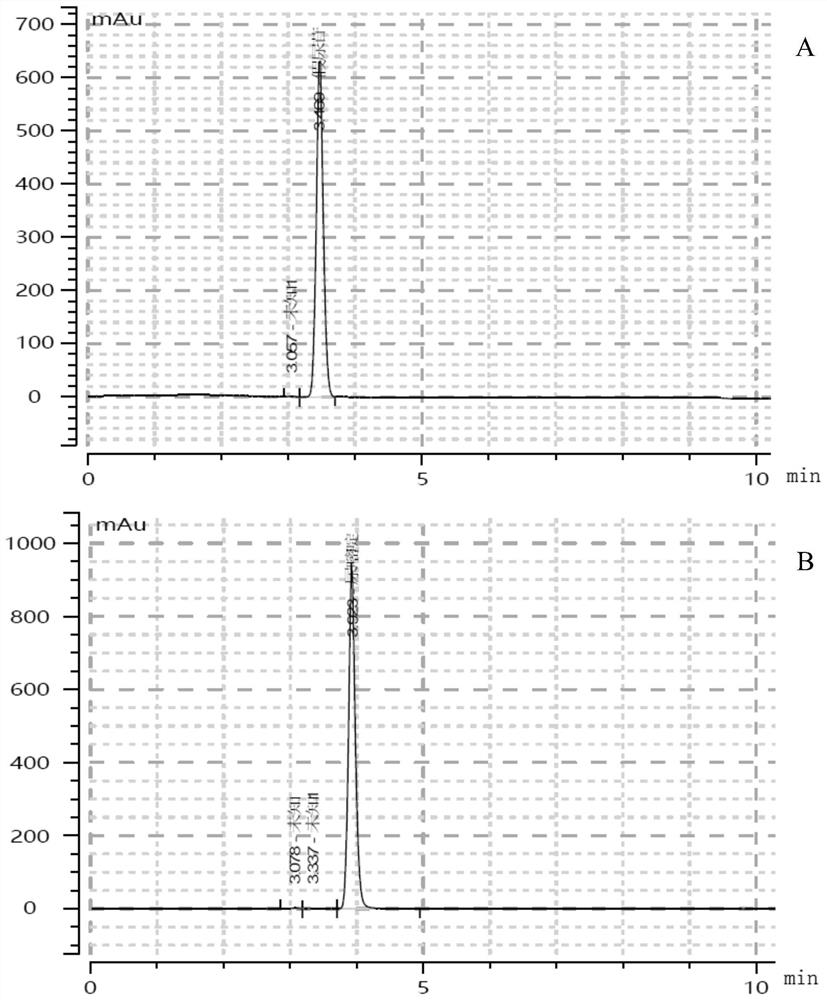
[0046]Pour the prepared two solutions into a 20L reactor, set the stirring speed at 150rpm, the temperature at 37°C, and the pH at 7.5. When the temperature rises to 37°C and the pH is 7.5 (±0.05), respectively add pseudouridine-5-phosphoglucosidase and its mutants at a final concentration of 0.5 mg / ml, and use 2M NaOH solution to automatically adjust the pH. After reacting for 30 minutes, slowly add 4.8 L of 100 mM completely dissolved uracil solution. After 2 hours, take 500 μL samples every 30 minutes and heat in a boiling water bath for 10 minutes, centrifuge and take the supernatant for dephosphorylation treatment and HPLC detection, as shown in figure 1 As shown, the reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com