Application of Hyperforcinol K in preparation of medicine for preventing and treating non-alcoholic fatty liver disease
A fatty liver disease, non-alcoholic technology, applied in the field of medicine, can solve the problems that have not been reported in the literature, and achieve the effect of reducing the accumulation of lipid droplets and reducing the number of lipid droplets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The extraction process of embodiment 1 Hyperforcinol K
[0037] 1. Experimental method
[0038]The Hyperforcinol K used in the present invention is extracted and separated from the traditional Chinese medicine Hypericum beanii, specifically: use 20 kg of Hypericum beanii as raw material, grind and extract 3 times with 70 L of 95% ethanol cold soaking, and combine 3 times The extract was concentrated to dryness under reduced pressure to obtain 1.8 kg of extract. The extract was suspended in 1.5 L of water and then extracted with petroleum ether for 5 extractions with a solvent volume of 4 L each time to obtain the petroleum ether fraction extract (506.0 g). After dissolving the petroleum ether part extract and mixing the sample, load the sample at a ratio of 3:1 (weight of silica gel filler: weight of concentrate), and separate by normal pressure normal phase silica gel column chromatography, with volume ratios of 100:1 and 10:1, respectively. 1, 5:2, 1:1, 1:5 mixed so...
Embodiment 2
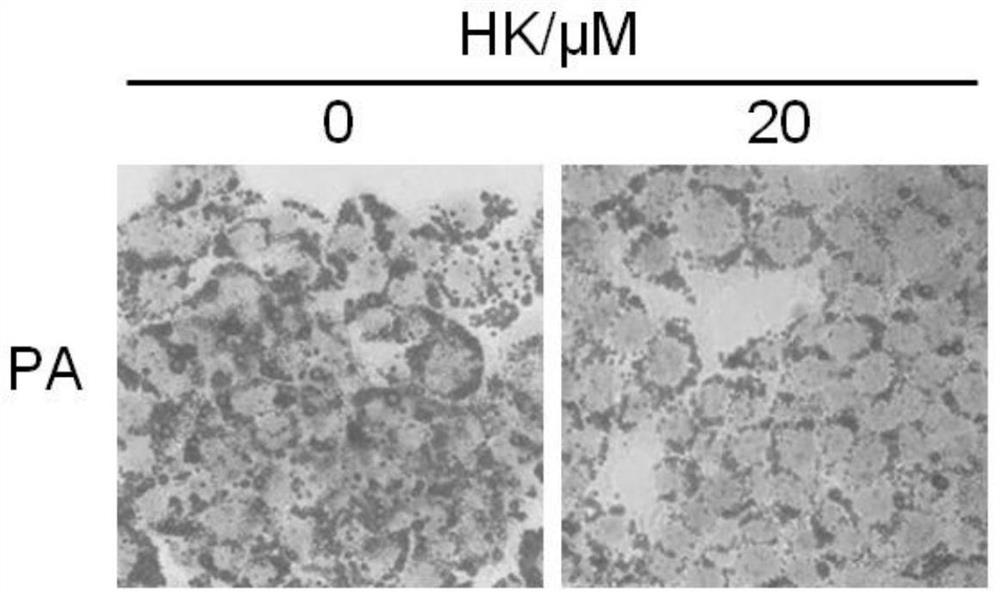
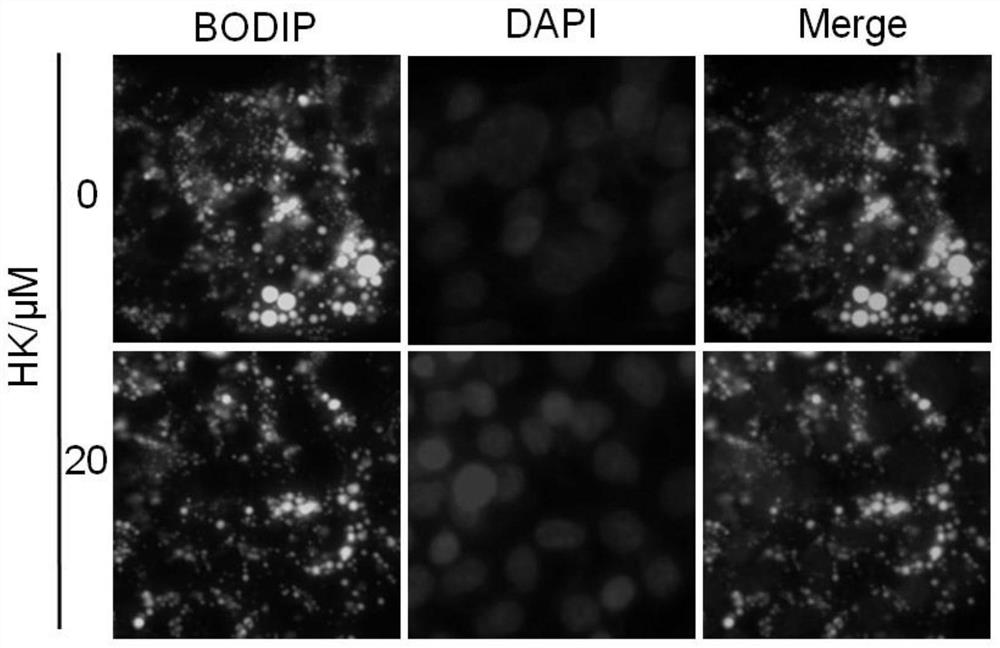
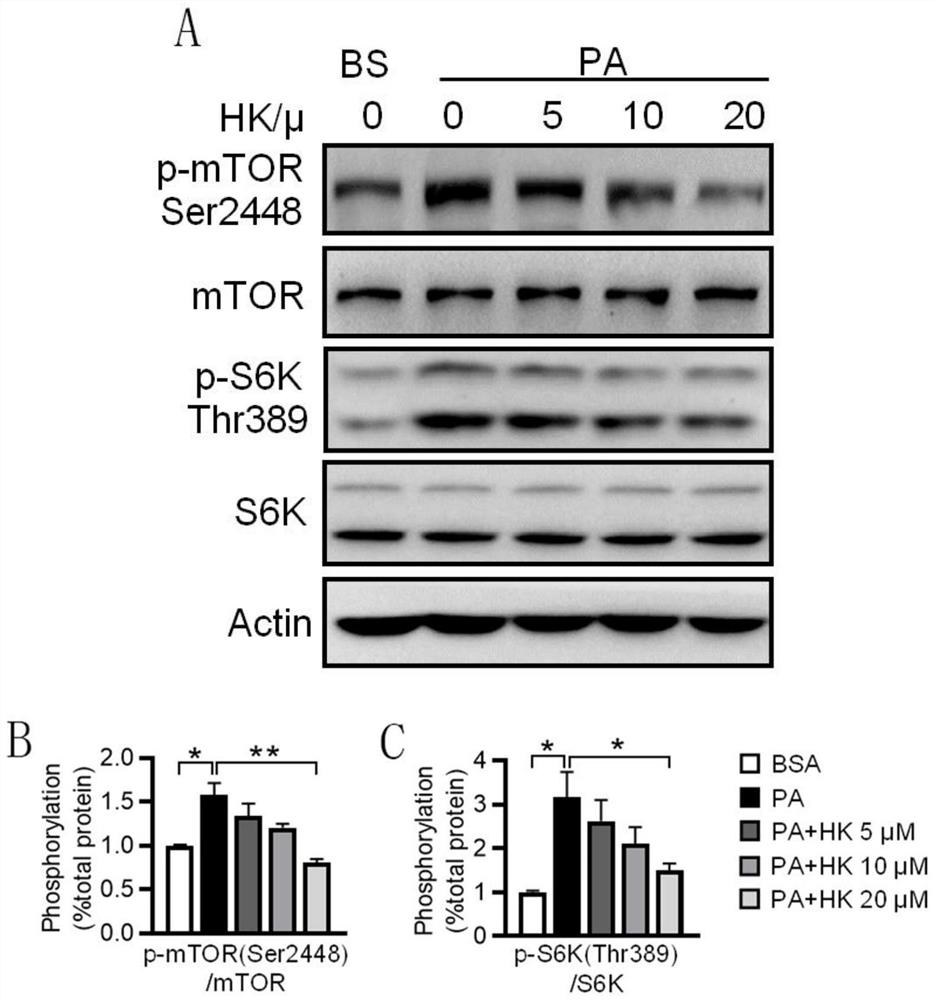
[0041] Example 2 Protective effect of HK on HepG2 cell injury induced by palmitic acid (PA)
[0042] 1. Experimental method
[0043] 1.1 Lipid medium preparation
[0044] Use PBS to prepare 20% d-BSA (fatty acid-free BSA) solution; use 0.1M NaOH solution to prepare 20mM PA solution, dissolve it in a water bath at 70-75°C, and fully saponify; take an equal volume of d-BSA solution and quickly add it to keep warm In the PA solution, it can be placed in a 50-55°C water bath to help dissolve, and a clear 10mM PA+10%d-BSA solution can be obtained, which can be filtered and sterilized in an ultra-clean bench to become palmitic acid lipid medium (PA stock solution) , Store at 4°C after aliquoting. The control solution is a solution obtained by mixing 20% d-BSA solution and an equal volume of 0.1M NaOH solution (BSA stock solution) after filter sterilization.
[0045] 1.2 Construction of PA-induced HepG2 cell injury model
[0046] Take PA and BSA stock solutions and add them to ...
Embodiment 3
[0053] Effect of Example 3 HK on the NASH mouse model induced by high-fat diet (HFD)
[0054] 1. Experimental method
[0055] 1.1 Construction method of NASH mouse model
[0056] Twenty-one C57BL / 6J male mice aged 6-8 weeks were randomly divided into three groups, namely the model group (HFD+solvent), the positive drug group (HFD+OCA) and the administration group (HFD+HK). 7 only. The mice in the three groups were all fed HFD at the age of 8 weeks, for a total of 12 weeks; at the age of 16 weeks, the mice were given intragastric administration, the model group was given intragastric administration of solvent olive oil, and the positive drug group was given intragastric administration of 10 mg / kg of OCA. The drug group was given 20 mg / kg of HK by intragastric administration for 4 weeks in total.
[0057] 1.2 Sample collection
[0058] After the model expired, the mice were fasted for 6 hours, and the whole blood of the mice was taken by picking the eyeballs. The blood colle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com