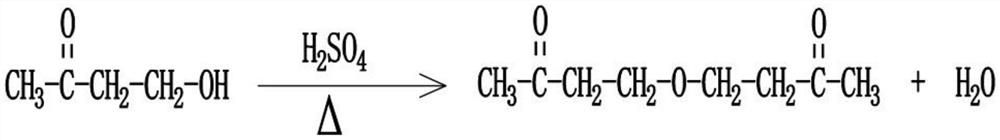
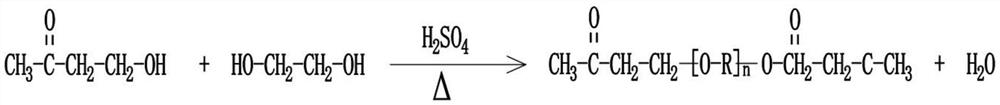
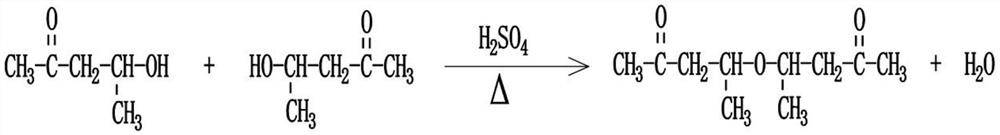Beta-polyether amine, synthesis method thereof and aliphatic polyurea coating containing beta-polyether amine
A synthesis method and polyetheramine technology, which are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of inability to use manual work, high input cost, and high input cost of raw materials, and ensure the opening time and construction. Simple process, good leveling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of β-polyetheramine:
[0046] (1) Etherification
[0047] Choose butanone alcohol as the raw material, add butanone alcohol and concentrated sulfuric acid into the reaction kettle, and use concentrated sulfuric acid as the catalyst in a sufficient amount. After mixing evenly, let it rest for more than 48 hours, and then heat it to 140-150°C, preferably 140°C. In the actual operation, the temperature is controlled to fluctuate within the range of ±2°C. After the reaction is completed, the reaction product is sequentially purified by alkali washing, water washing and distillation;
[0048] Among them, in the alkali washing step, sodium carbonate or sodium hydroxide is used to react at room temperature, and neutralize the acid substances in the reaction product until the pH of the reaction product=7; in the water washing step, the aforementioned alkali washing step The generated salts and other impurities are removed; the water in the washed reaction product i...
Embodiment 2
[0055] Aliphatic polyurea coating S1 was prepared.
[0056] (1) Component A, in parts by mass, includes the following components:
[0057] 467 parts of diphenylmethane diisocyanate (MDI 50, purchased from Yantai Wanhua)
[0058] 452 parts of polyether polyols (Tdiol 2000, purchased from Tianjin Petrochemical);
[0059] Mix MDI50 and Tdiol 2000 uniformly at first, carry out pre-polymerization, and react for 4 hours at 85°C.
[0060] (2) B component, in parts by mass, includes the following components:
[0061]
[0062] Put the above components into the reaction kettle, vacuumize, mix evenly, heat to 90-100°C, and react for 2 hours to obtain component B.
[0063] (3) Preparation of aliphatic polyurea coating
[0064] Mix the above-mentioned components A and B according to the mass ratio of 1:1 to obtain the aliphatic polyurea coating, which is marked as S1.
[0065] At 25°C, the aliphatic polyurea coating S1 was tested for performance. The pot life was 20 minutes, the vi...
Embodiment 3
[0068] Aliphatic polyurea coating S2 was prepared.
[0069] (1) Component A, in parts by mass, includes the following components:
[0070] Diphenylmethane diisocyanate (MDI 100) 220 parts
[0071] Diphenylmethane diisocyanate (MDI 50) 220 parts
[0072] 426 parts of polyether polyol (Tdiol 2000);
[0073] The above-mentioned components were firstly mixed uniformly, pre-polymerized, and reacted at 85°C for 4 hours.
[0074] (2) B component, in parts by mass, includes the following components:
[0075]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com