Indobufen-containing pharmaceutical composition and preparation method thereof
A technology for indobufen and a composition, applied in the field of pharmaceutical preparations, can solve the problems of difficulty, inaccurate curative effect, large dosage of preparation excipients, etc., and achieve the effects of improving taking compliance, reducing the variety of excipients, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
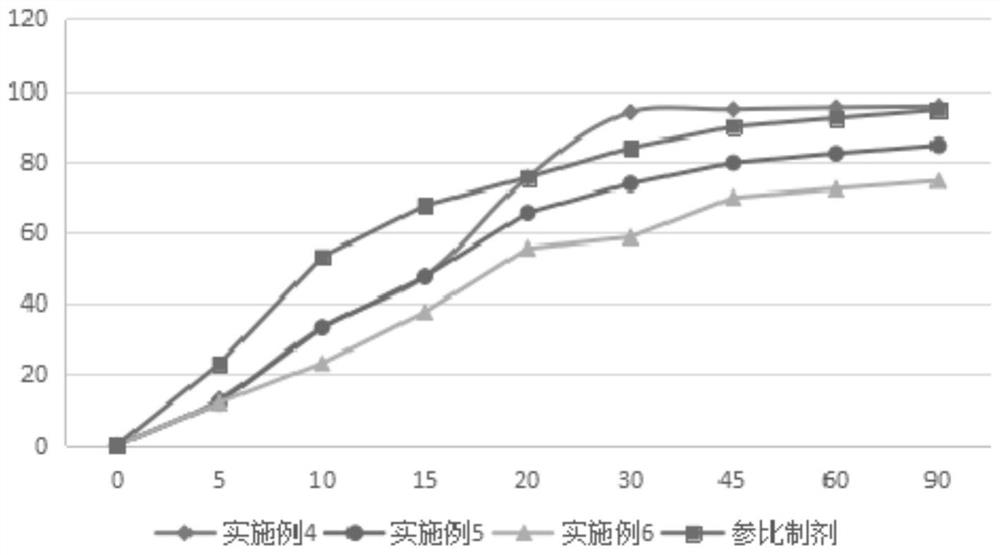
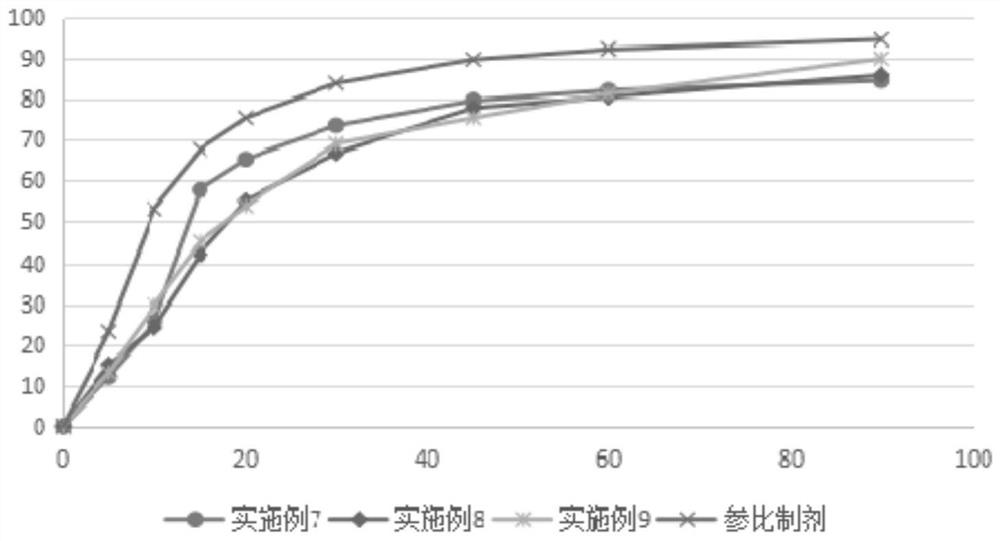
Examples
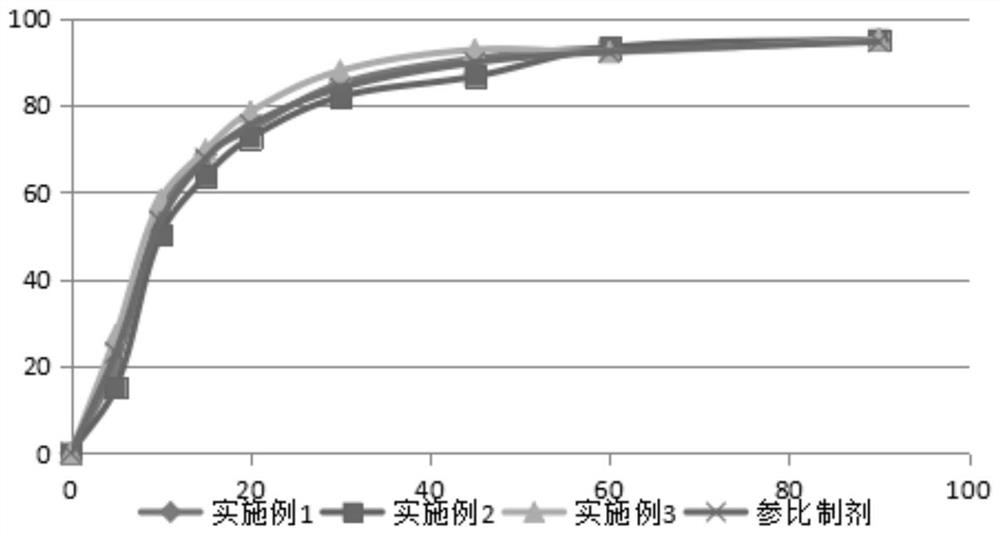
Embodiment 1
[0039] Table 1
[0040]
[0041] Preparation
[0042] (1) Preparation of preparation composition: Prescribed amount of polyethylene glycol 6000 (manufacturer: Hubei Gedian Renfu Pharmaceutical Excipients Co., Ltd., the same below) and xylitol (manufacturer: Hunan Jiudian Pharmaceutical Co., Ltd., the same below) ) in a stainless steel container with stirring, heated to 80°C, added indobufen (manufacturer: Zhejiang Tiantai Fuda Pharmaceutical Co., Ltd., the same below) that meets the particle size requirement (D90 is 25 μm) and stirred for half an hour, then cooled , 80 meshes of the cooled composition are mechanically pulverized and prepared (hammer mill, manufacturer: Shanghai Yinglu Machinery Equipment Co., Ltd., the same below) into a composition; Anhui Shanhe, the same below) are mixed uniformly to form a preparation composition; (2) tableting (tablet press, manufacturer: Beijing Sinopharm Longli Technology Co., Ltd.): the preparation composition adds 1% lubricant magn...
Embodiment 2
[0044] Table 2
[0045]
[0046]
[0047] Preparation
[0048] (1) Preparation of preparation composition: Place the prescription amount of polyethylene glycol 6000 (manufacturer: Hubei Gedian Renfu Pharmaceutical Excipients Co., Ltd.) and xylitol in a stainless steel container with stirring, heat to 80°C, add Indobufen with a particle size requirement of D90 of 20 μm was stirred for half an hour, then cooled, and the cooled composition was mechanically crushed to 80 mesh to prepare a composition; the composition was added to the prescription amount of pregelatinized starch and mixed evenly to form a preparation combination (2) tabletting: preparation composition adds 1% lubricant magnesium stearate and is prepared into tablet, and sheet weight is about 263mg, and hardness 4kgf.
Embodiment 3
[0050] table 3
[0051]
[0052] Preparation
[0053] (1) Preparation of preparation composition: Place the prescription amount of polyethylene glycol 4000 (manufacturer: Hubei Gedian Renfu Pharmaceutical Excipients Co., Ltd.) and xylitol in a stainless steel container with stirring, heat to 80°C, add Indobufen with a particle size requirement of D90 of 30 μm was stirred for half an hour, then cooled, and the cooled composition was mechanically pulverized to 80 mesh to prepare a composition; the composition was added to the prescription amount of pregelatinized starch and mixed evenly to form a preparation combination (2) tabletting: preparation composition adds 1% lubricant magnesium stearate to be prepared into tablet, and sheet weight is about 364mg, and hardness 7kgf.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com