RCAN1.4 promoter fragment for cell hypoxia and HIF1alpha protein activity indicator and application of RCAN1.4 promoter fragment
A technology of RCAN1.4 and promoter, applied in the field of genetic engineering, can solve the problems of needing four hours to overnight incubation, unstable results, high detection cost, etc., to avoid error rate and sample loss, convenient and sensitive detection, and reduce protein degradation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 plasmid construction
[0049] 1.1 Construction of RCAN1.4 promoter region RCAN1.4-315-15-LUC and RCAN1.4-315-15-GFP plasmids:
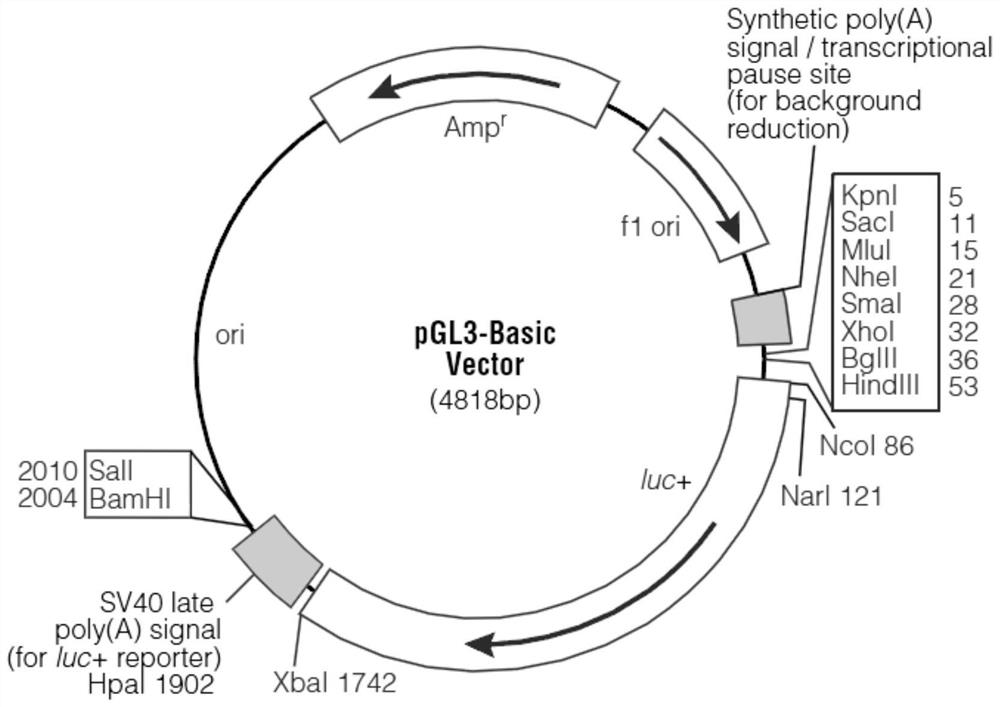
[0050] The first ATG of the first exon of RCAN1.4 was named +1, and the sequence of RCAN1.4 promoter region -315bp-15bp was inserted into the multiple cloning sites SmaI and HindIII of pGL3-basic to obtain RCAN1. 4-315-15-LUC plasmid. Under normal circumstances, the pGL3-basic vector contains the coding sequence for expressing the luciferase reporter gene LUC and has no promoter element, and cannot initiate the expression of the LUC gene. If the inserted exogenous sequence has promoter activity, it is a promoter element , then the luciferase activity of the reporter vector will increase, so the luciferase activity of the plasmid depends on the promoter activity of the insert. We excise the LUC coding sequence in the RCAN1.4-315-15-LUC plasmid and replace it with the GFP coding sequence, then when the promoter activity of the inse...
Embodiment 2
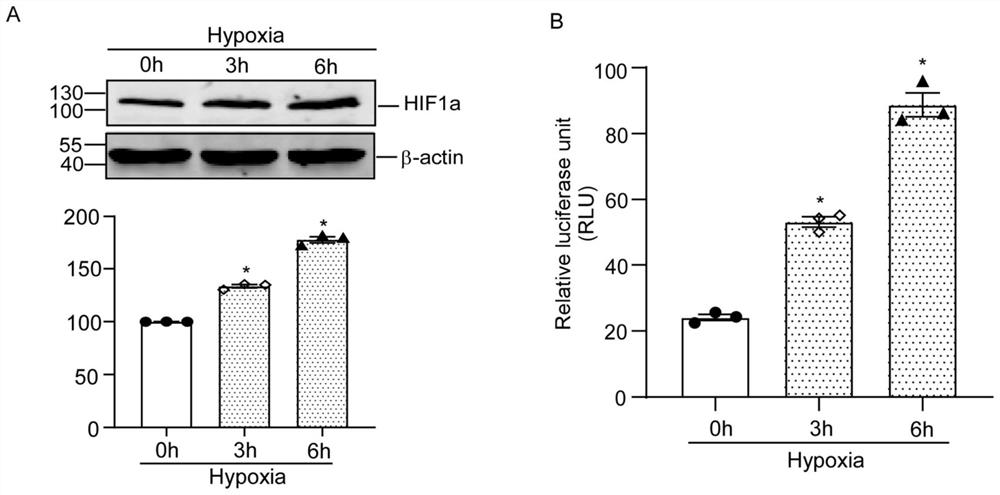
[0068] Example 2 Changes in HIF1α protein expression and luciferase activity of RCAN1.4-315-15-LUC under hypoxic conditions
[0069] 2.1 Transfect RCAN1.4-315-15-LUC into HEK293 cells using lipo3000 transfection reagent (or other brands of transfection reagents are acceptable), and the density of HEK293 cells is about 75% when transfected.
[0070] 2.2 48 hours after transfection, the cells were subjected to hypoxia (1% O 2 ) after the treatment, discard the medium immediately, wash the cells with pre-cooled PBS, add 100 μL of 1×passive lysis buffer cell lysate, place on a shaker at room temperature for lysis for 20 min until the cells are completely lysed, and transfer the cell lysate to In 1.5mL of EP, vortex shaker to mix for 15 seconds, centrifuge at 12000g for 5min at 4°C, take the supernatant and put it on ice for testing.
[0071]2.3 Using the Dual Luciferase Reporter Gene Detection Kit, add 2 μL of cell supernatant to a new EP tube in sequence, then add 10 μL of Firef...
Embodiment 3
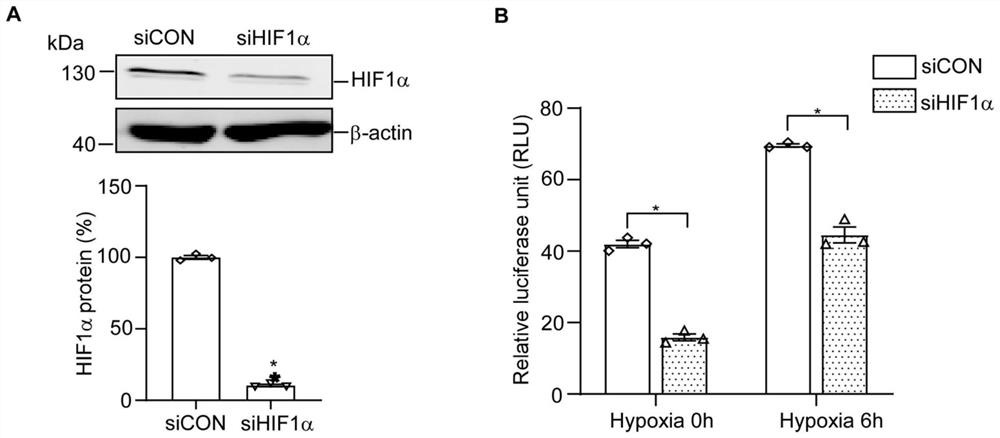
[0074] Example 3 Changes in luciferase activity of RCAN1.4-315-15-LUC after knocking down HIF1α protein under hypoxic conditions
[0075] 3.1 Use lipo3000 transfection reagent (or other brands of transfection reagents are acceptable) to co-transfect the constructed RCAN1.4-315-15-LUC plasmid with siHIF1α or siCON into HEK293 cells in a 48-well culture plate. The cell density was 75%.
[0076] 3.2 48 hours after transfection, the cells were subjected to hypoxia (1% O 2 ) after the treatment, discard the medium immediately, wash the cells with pre-cooled PBS, add 100 μL of 1×passive lysis buffer cell lysate, place on a shaker at room temperature for lysis for 20 min until the cells are completely lysed, and transfer the cell lysate to In 1.5mL of EP, vortex shaker to mix for 15 seconds, centrifuge at 12000g for 5min at 4°C, take the supernatant and put it on ice for testing.
[0077] 3.3 Using the Dual Luciferase Reporter Gene Detection Kit, add 2 μL of cell supernatant to a n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com