Preparation method of zolpidem and key intermediate thereof
A technology for zolpidem and intermediates, applied in the field of pharmaceutical synthesis, can solve the problems of unstable yield, high production equipment requirements, and high production costs, achieve high reaction yield and purity, improve operational safety, and reduce production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
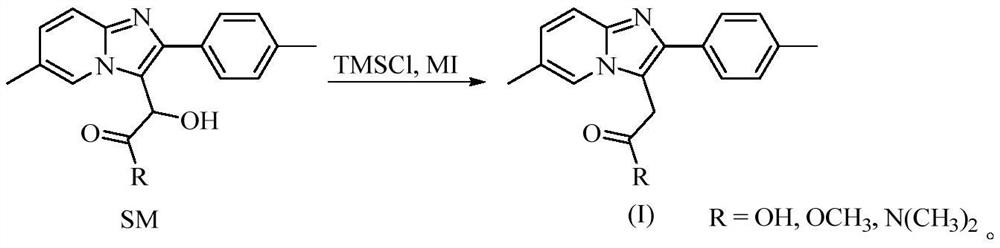
[0059] Under argon protection, 2-hydroxy-N,N-dimethyl-2-(6-methyl-2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)acetamide [R=N(CH 3 ) 2 , named as SM-1, 32.34g, 0.1mol], trimethylchlorosilane (TMSCl, 54.32g, 0.5mol), sodium iodide (74.95g, 0.5mol) were added in dry acetonitrile (300ml), temperature controlled 55 ℃ to after the end of the reaction, the reaction solution was down to room temperature, added in purified water (800ml), extracted with dichloromethane (300ml × 3), the organic phase was washed with saturated sodium thiosulfate solution (300ml × 3), purified water ( 300ml×2), washed with saturated brine (300ml×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain zolpidem with a yield of 96.4% and a HPLC purity of 99.892%.
Embodiment 2
[0061] Under argon protection, 2-hydroxy-N,N-dimethyl-2-(6-methyl-2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)acetamide [R=N(CH 3 ) 2 , SM-1, 32.34g, 0.1mol], trimethylchlorosilane (TMSCl, 43.46g, 0.4mol), sodium iodide (59.96g, 0.4mol) were added to dry acetonitrile (300ml), and the temperature was controlled at 60°C After the reaction was finished, the reaction solution was cooled to room temperature, added in purified water (800ml), extracted with chloroform (300ml×3), the organic phase was washed with saturated sodium thiosulfate solution (300ml×3), purified water (300ml×2 ), washed with saturated brine (300ml×2), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain zolpidem with a yield of 95.9% and an HPLC purity of 99.873%.
Embodiment 3
[0063] Under argon protection, 2-hydroxy-N,N-dimethyl-2-(6-methyl-2-(p-tolyl)imidazo[1,2-a]pyridin-3-yl)acetamide [R=N(CH 3 ) 2 , SM-1, 32.34g, 0.1mol], trimethylchlorosilane (TMSCl, 38.02g, 0.35mol), sodium iodide (52.46g, 0.35mol) were added to dry acetonitrile (300ml), and the temperature was controlled at 55°C After the reaction was finished, the reaction solution was cooled to room temperature, added in purified water (800ml), extracted with ethyl acetate (300ml×3), the organic phase was washed with saturated sodium thiosulfate solution (300ml×3), purified water (300ml×3) 2) Washing, washing with saturated brine (300ml×2), drying over anhydrous sodium sulfate, filtering, and concentrating the filtrate to dryness under reduced pressure to obtain zolpidem with a yield of 92.9% and a HPLC purity of 99.791%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com