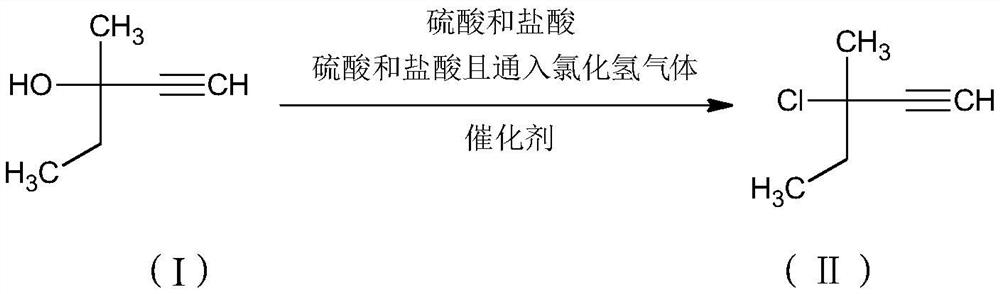
Preparation method and application of 3-methyl-3-amino-1-pentyne
A technology of methyl and pentyne, which is applied in the field of preparation of 3-methyl-3-amino-1-pentyne, can solve the problems of difficulty in layering, low yield and large amount, and achieves convenient process implementation and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
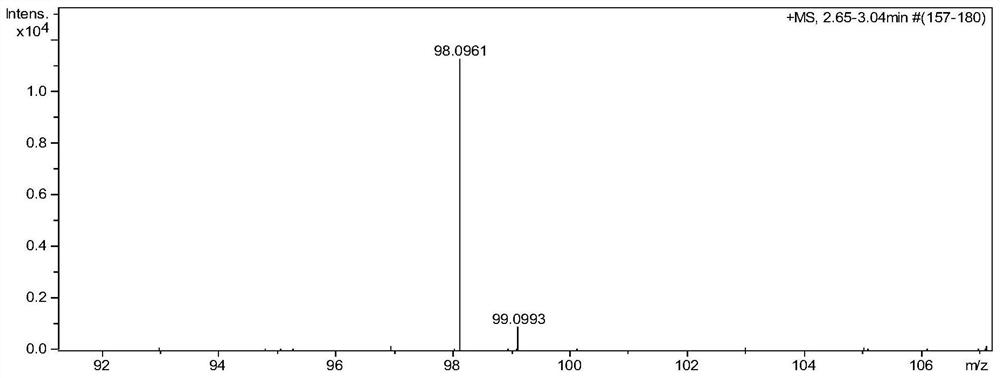
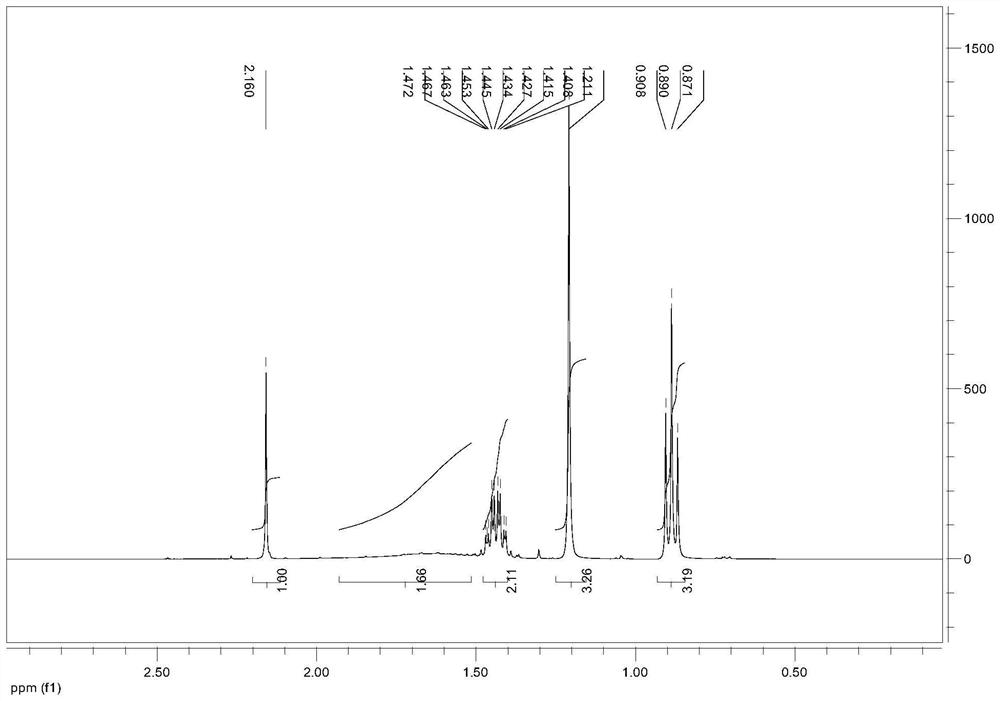
[0040] Example 1 Preparation of 3-methyl-3-chloro-1-pentyne
[0041] Put 90g of 30% industrial hydrochloric acid (0.74mol) into the reaction bottle, start stirring, cool down to -5°C in a cold bath, add 3g of catalyst cuprous chloride, control the temperature at -5°C to 5°C and add to the solution for 2 hours 60 g (0.60 mol) of 3-methyl-1-pentyn-3-ol raw material and 80 g (0.80 mol) of 98% concentrated sulfuric acid were added dropwise, and then kept at -5°C to 5°C for 2 hours.
[0042] After the reaction system was left to stand and separated, 73 g of the upper oil layer and 160 g of the lower acid layer were obtained. The oil layer was washed with 20 g of water to obtain 70 g of the product, namely 3-methyl-3-chloro-1-pentyne.
[0043] The content of the obtained product was confirmed to be 97% by GC detection (area normalization method), and the calculated yield was 95.3%.
Embodiment 2
[0044] Example 2 Preparation of 3-methyl-3-chloro-1-pentyne
[0045] Carry out identically with embodiment 1, difference is to replace cuprous sulfate with catalyst cuprous chloride, other conditions and technological operation are unchanged. As a result, 68 g of the product 3-methyl-3-chloro-1-pentyne were obtained.
[0046] GC detection confirmed that the product content was 97%, and the calculated yield was 92.6%.
Embodiment 3
[0047] Example 3 Preparation of 3-methyl-3-chloro-1-pentyne
[0048] Proceed in the same manner as in Example 1, except that the catalyst cuprous chloride is replaced by silver nitrate, and other conditions and process operations remain unchanged to obtain 67g of product 3-methyl-3-chloro-1-pentyne.
[0049] GC detection confirmed that the product content was 97%, and the calculated yield was 91.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com