Preparation method and application of dibenzothienyl lanthanide metal organic framework compound
A dibenzothienyl lanthanum and metal organic framework technology, which is applied in the direction of organic compound/hydride/coordination complex catalyst, organic chemistry, chemical instruments and methods, etc., can solve the difficulty of increasing process flow, unfavorable purification and separation, Catalysts are easy to deactivate and other problems, and achieve good industrialization prospects, easy separation and purification, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
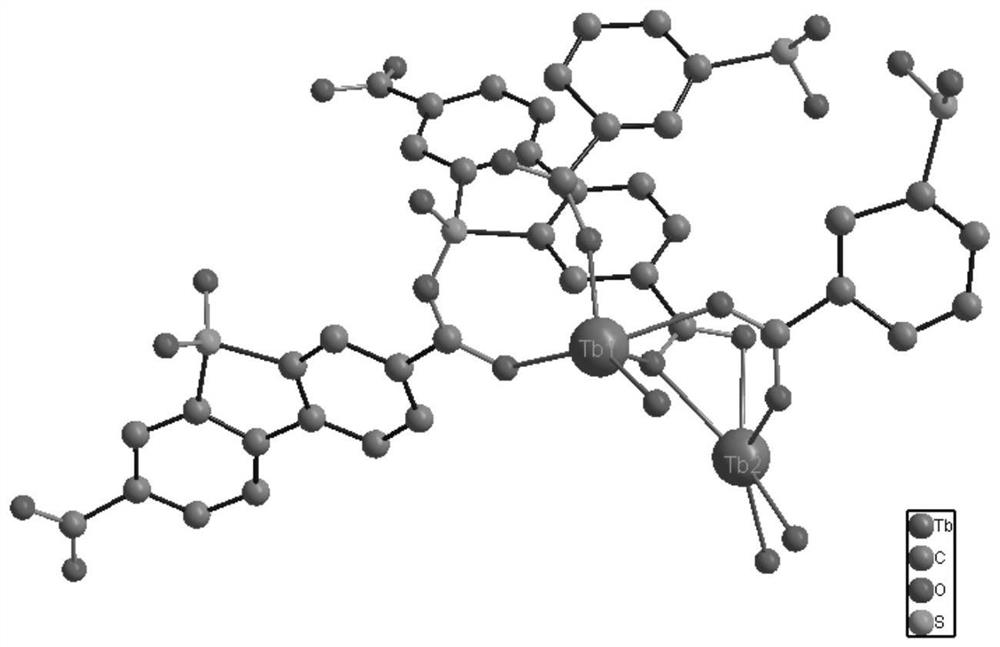
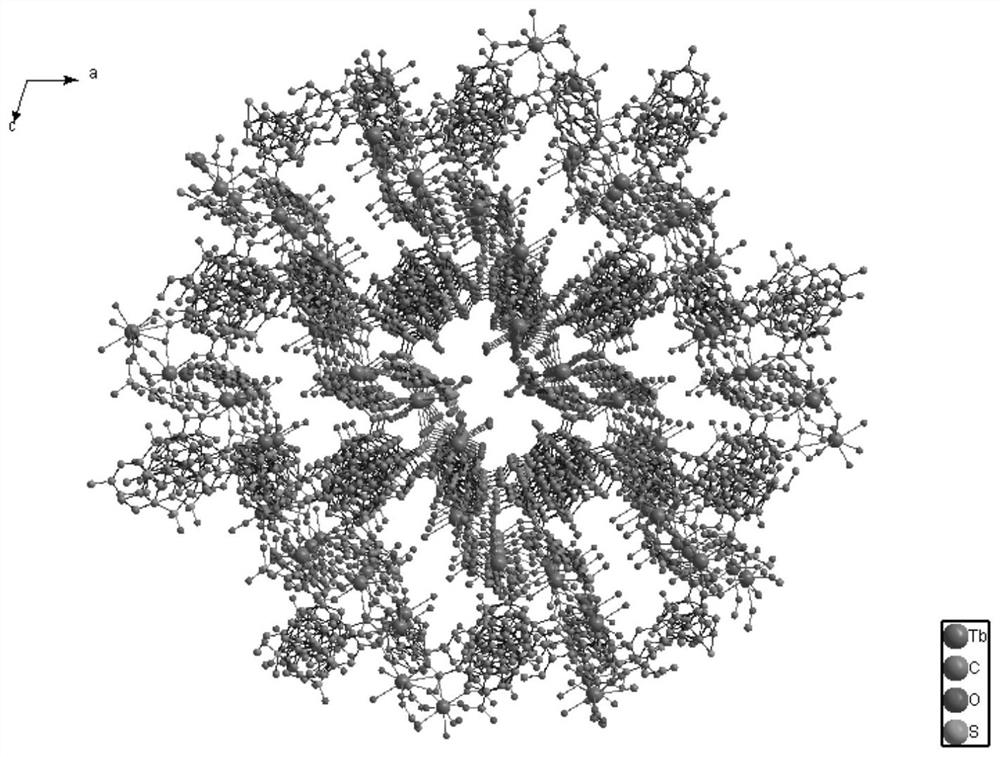
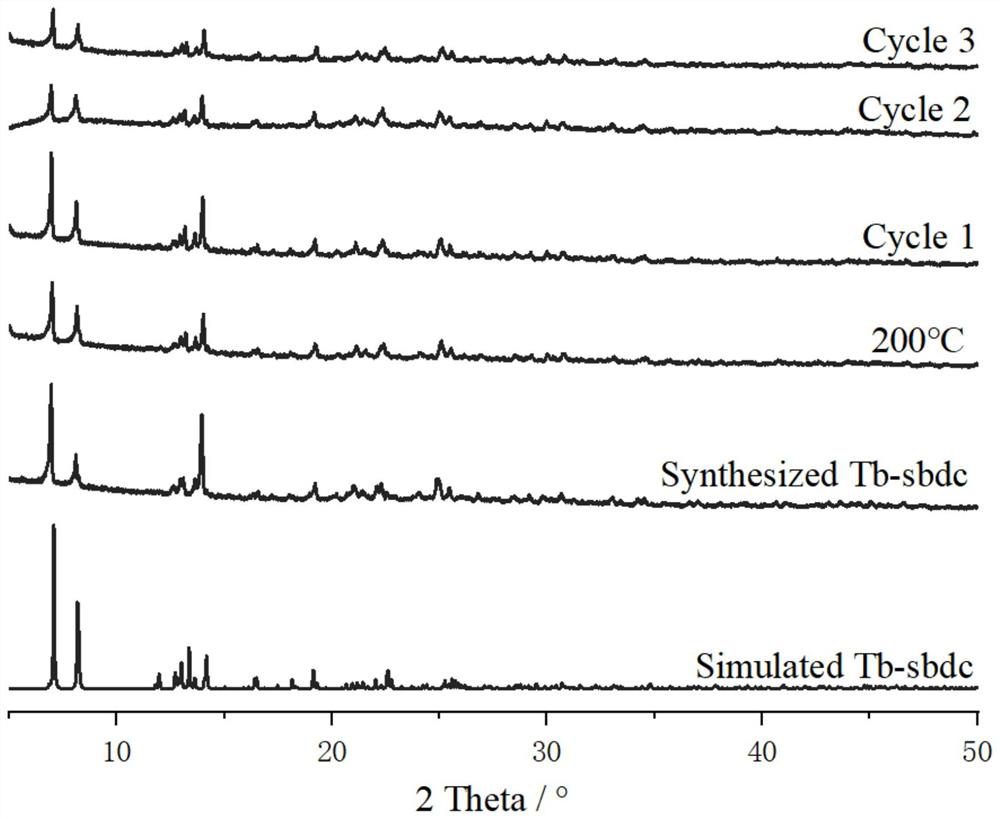
[0029] Add Ligand H to 5 small empty vials respectively 2 sbdc (0.091g, 0.3mmol) and Tb (NO 3 ) 2 ·6H 2 O (0.091g, 0.2mmol), then add 5mL ethanol and 1mL deionized water to these 5 vials respectively, stir evenly, then place these 5 vials in an oven, burn at 140°C for 96h, close the oven, and cool At room temperature, colorless cubic crystals were produced, filtered, and dried to obtain the target material Tb-sbdc, about 380 mg was obtained from 5 vials, and the yield was about 60%. Molecular formula: C 42 h 24 o 21 S 3 Tb2. Elemental analysis (%) Found: C 37.31%, H 2.679%. The crystal structure diagram of the target material Tb-sbdc obtained, such as figure 1 As shown, the 3D structure schematic diagram, such as figure 2 As shown in the XRD diagram, such as image 3 As shown, the thermogravimetric analysis diagram, such as Figure 4 As shown, the catalytic cycle utilization diagram, such as Figure 11 shown.
Embodiment 2
[0031] Add Ligand H to 5 small empty vials respectively 2 sbdc (0.091g, 0.3mmol) and TbCl 3 ·6H 2O (0.075g, 0.2mmol), then add 5mL ethanol and 1mL deionized water to these 5 vials respectively, stir evenly, then place these 5 vials in an oven, burn at 140°C for 96h, close the oven, and cool At room temperature, colorless cubic crystals were produced, filtered and dried to obtain the target material Tb-sbdc, about 370 mg was obtained from 5 vials, and the yield was about 58%. Molecular formula: C 42 h 24 o 21 S 3 Tb 2 . Elemental analysis (%) Found: C 37.31%, H2.679%. The crystal structure diagram of the target material Tb-sbdc obtained, such as figure 1 As shown, the 3D structure schematic diagram, such as figure 2 As shown in the XRD diagram, such as image 3 As shown, the thermogravimetric analysis diagram, such as Figure 4 As shown, the catalytic cycle utilization diagram, such as Figure 11 shown.
Embodiment 3
[0033] 10 mg Tb-sbdc was weighed and soaked in a solution of 2',7'-dichlorofluorescein in dichloromethane for 24 hours, then the product was filtered and washed with 10 mL of dichloromethane to obtain a solid. Then the solid is dissolved with 100 microliters of concentrated hydrochloric acid, and this solution is constant volume in a 50mL volumetric flask with methanol, and the dye adsorption ultraviolet curve of the prepared solution is measured, as shown in Figure 5 shown. Comparing the UV absorption standard curve of fluorescein, the mass ratio of Tb-sbdc to fluorescein was calculated to be 6.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com