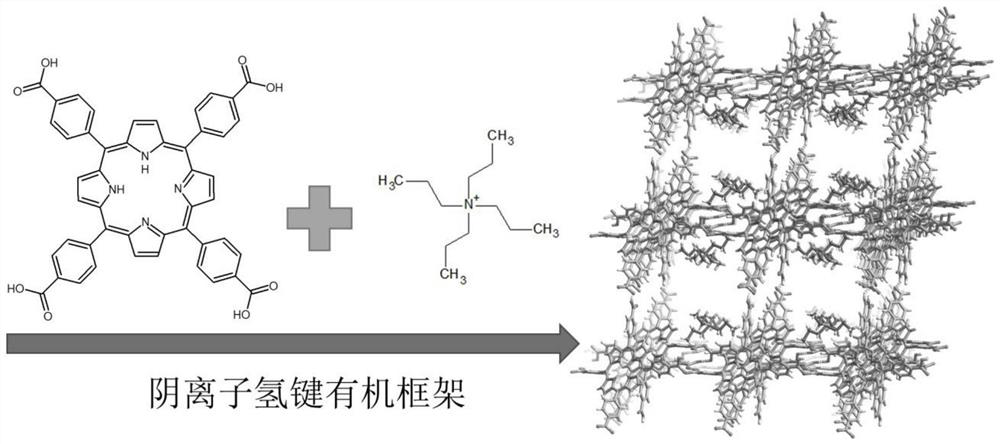
Anion hydrogen bond organic framework material based on carboxylic acid monomer as well as preparation method and application of anion hydrogen bond organic framework material
A technology of organic framework and carboxylic acid monomer, which is applied in the field of anionic hydrogen bond organic framework materials, and can solve problems such as no systematic strategy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Anionic hydrogen-bonded organic framework material (PFC-33) based on 1,3,6,8-tetra(p-carboxyphenyl)porphyrin and tetrapropylammonium
[0084] Material preparation process: Dissolve 59 mg of 1,3,6,8-tetrakis(p-carboxyphenyl)porphyrin in 0.5 ml of nitrogen, nitrogen-dimethylacetamide, dissolve it by ultrasonication, add 5 ml of methanol, and then Add 70 microliters of 40% by mass fraction of tetrapropylammonium hydroxide aqueous solution, and sonicate for 5 minutes to obtain a clear purple-black solution. Let stand in the open at room temperature for 2 days. With the volatilization of methanol, PFC-33 will gradually precipitate out. After washing and centrifuging with acetone three times, pure PFC-33 can be obtained (this material can still be obtained by expanding the formula by the same amount).
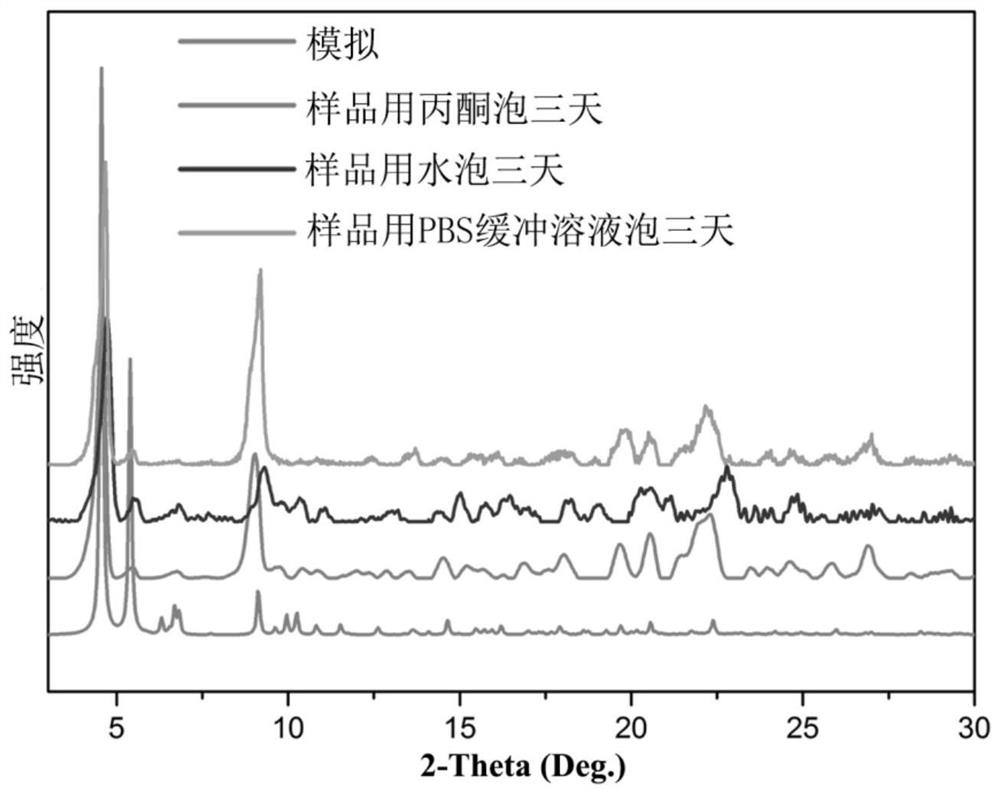
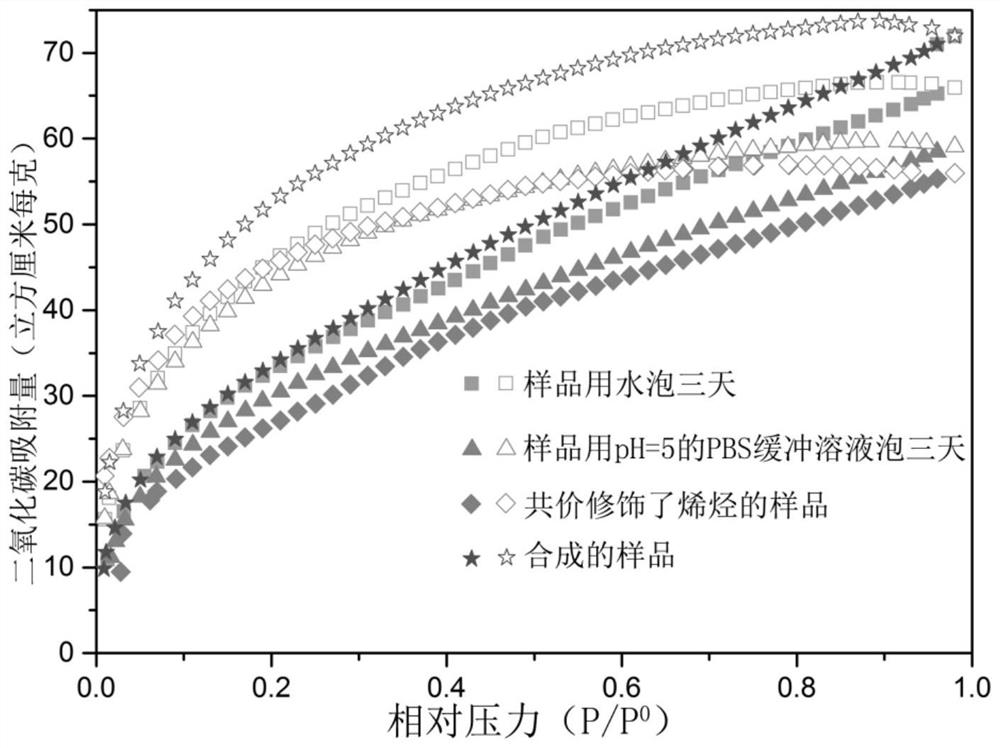
[0085] figure 2 The powder X-ray diffraction spectrum, in which the retention of the diffraction peaks proves that the crystallinity of the sample will not be dama...
Embodiment 2
[0086] Example 2 Preparation of anionic hydrogen-bonded organic framework materials based on 1,3,6,8-tetra(p-carboxyphenyl)porphyrin and tetramethylammonium, tetraethylammonium, tetrabutylammonium, ammonium ion ( PFC-33(TMA),PFC-33(TEA),PFC-33(TBA),PFC-33(NH4)
[0087] PFC-33 (TMA), PFC-33 (TEA), PFC-33 (TBA), the preparation process of PFC-33 (NH4) is identical with embodiment 1, only needs to replace tetrapropyl ammonium hydroxide with following concrete compound .
[0088]
Embodiment 3
[0089] Example 3 is based on 1,3,6,8-tetra(p-carboxyphenyl)porphyrin (Ni), 1,3,6,8-tetra(p-carboxyphenyl)porphyrin (Cu) and tetrapropylammonium Preparation of anionic hydrogen-bonded organic framework materials (PFC-33-Ni, PFC-33-Cu)
[0090] The preparation process of PFC-33-Ni and PFC-33-Cu is the same as that of Example 1, except that 1,3,6,8-tetrakis(p-carboxyphenyl)porphyrin is replaced by the following specific compounds.
[0091]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com