Stepped zinc aspartate coordination polymer as well as preparation method and application thereof
A technology of zinc aspartate and coordination polymer, applied in the directions of fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of weak ultraviolet absorption, small extinction coefficient, enhanced toxicity, etc., and achieves convenient and simple preparation process. The effect of good crystal quality and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 zinc coordination polymer
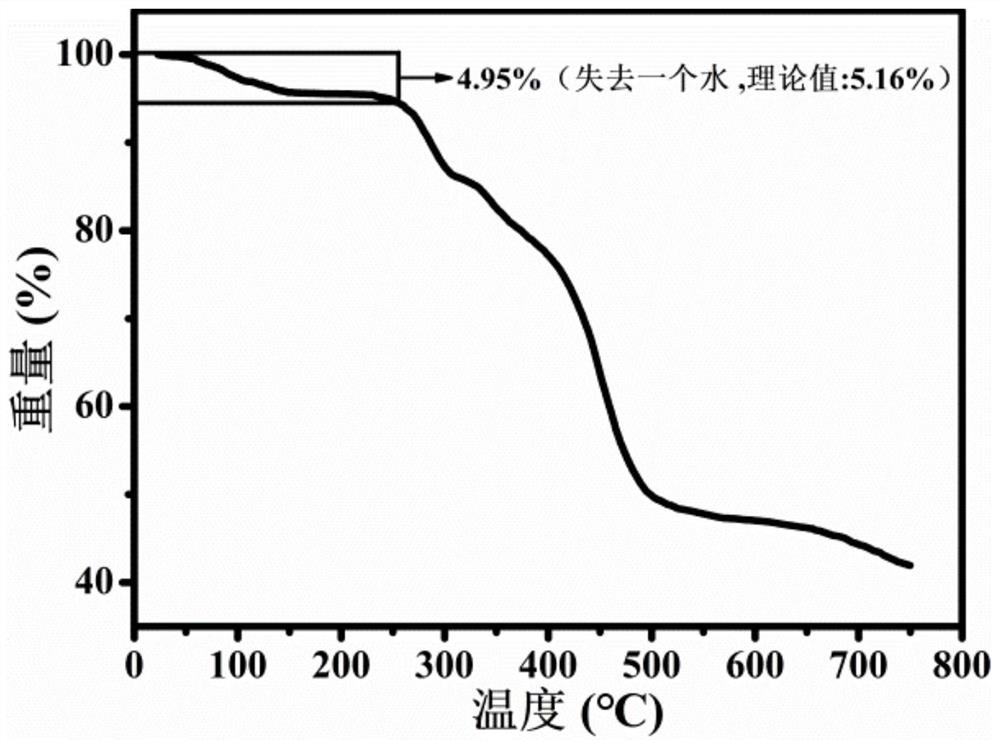
[0032] H 3 caa (13.80mg, 0.05mmol), ZnSO 4 ·7H 2 A mixture of O (28.70mg, 0.1mmol), distilled water (10.00mL) and 100μL NaOH (1mol / L) was added to a polytetrafluoroethylene tube with a capacity of 25mL. Under heating for 72 hours, then naturally cooled to room temperature. The colorless blocky crystals were collected by filtration, washed with water and dried in air with a yield of 60.41% (as Zn 2+ count). Elemental Analysis: C 12 h 12 NO 7 Zn (%) experimental value (theoretical value): C, 41.44 (41.46), H, 3.51 (3.48), N, 4.10 (4.03).
Embodiment 2
[0033] Example 2 Determination of Zinc Coordination Polymer Crystal Structure
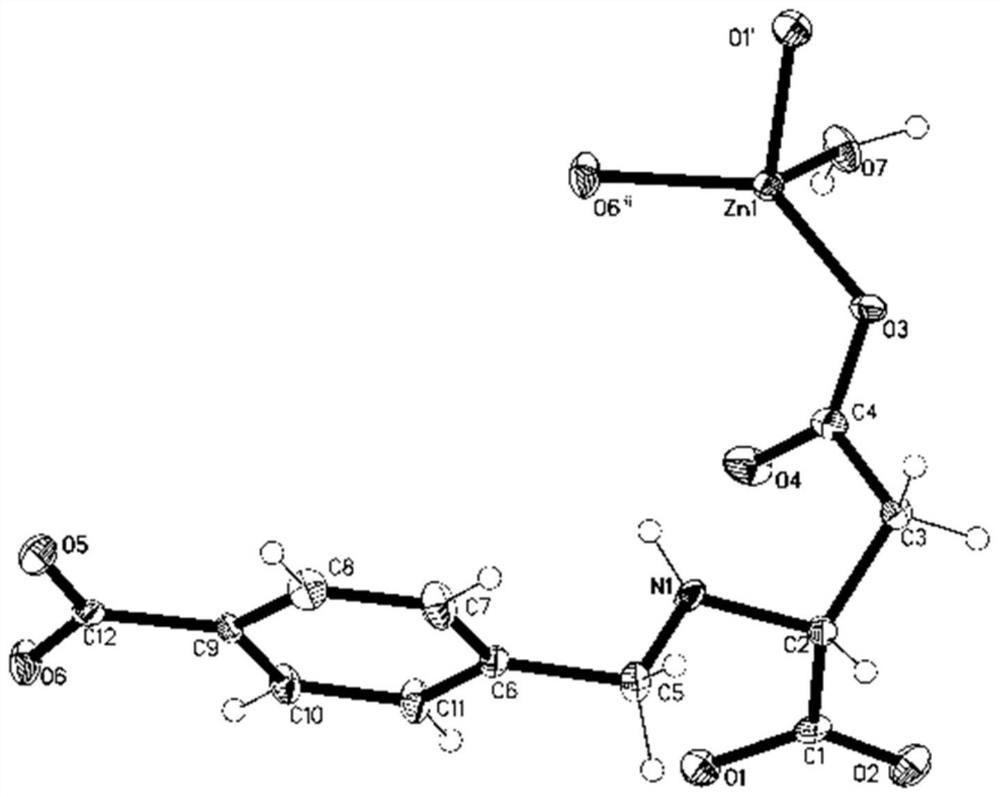
[0034] The crystal structure was determined by X-ray diffraction, the test instrument was Bruker D8Venture, the radiation source was Mo-Kα ray monochromated by graphite monochromator, the scanning method was rotation ω angle, and the diffraction data were collected at room temperature (296K). The original data were restored by the SAINT program and then corrected for absorption using the SADABS program. The crystal structure was solved by SHELXL-2014 direct method. The detailed crystal determination data are shown in Table 1, and the crystal structure is shown in figure 1 shown.
[0035] Table 1 Crystallographic data of zinc coordination polymers
[0036]
[0037]
Embodiment 3
[0038] Embodiment 3 powder diffraction analysis phase
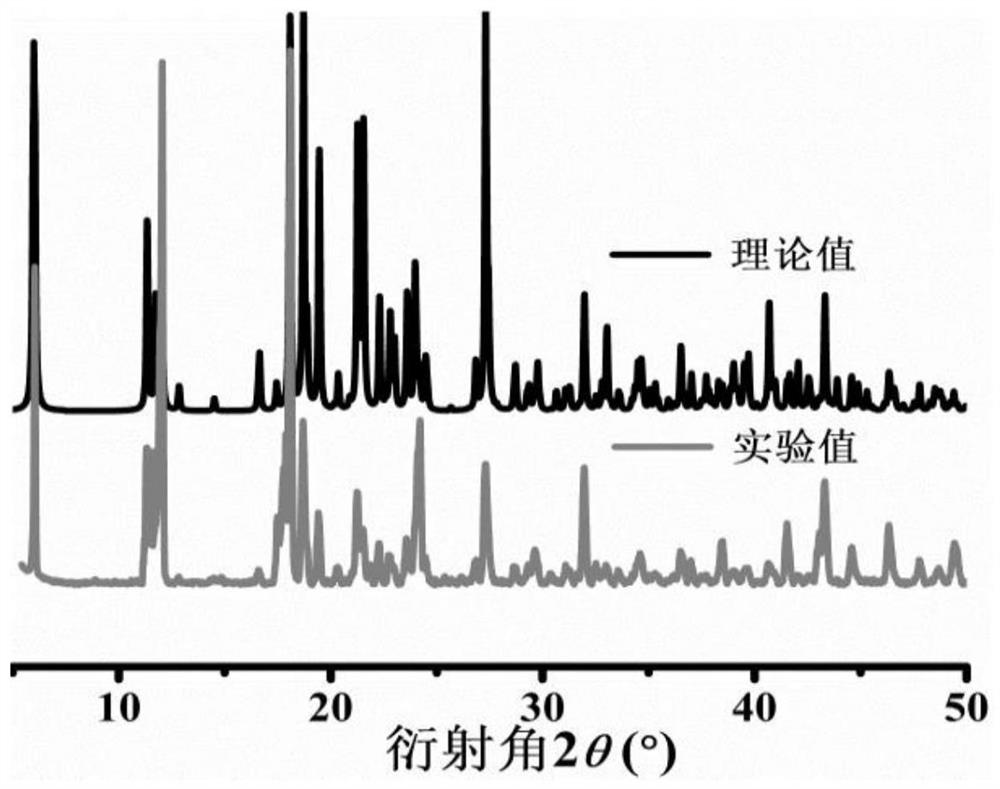
[0039] X-ray powder diffraction results show that the polycrystalline sample test value and the single crystal structure fitting value are almost consistent, showing that the zinc coordination polymer of the present invention is a pure phase, see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com