Chemical chain reaction system and method driven by high-nitrogen-content biomass
A biomass and high nitrogen content technology, applied in chemical instruments and methods, biofuels, inorganic chemistry, etc., can solve the problems of lack of energy coupling in the chemical chain ammonia synthesis system, high energy consumption in the air separation process, wear and tear of nitrogen carrier, etc. Achieve the effects of improving mechanical strength and wear resistance, realizing energy recovery and increasing service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
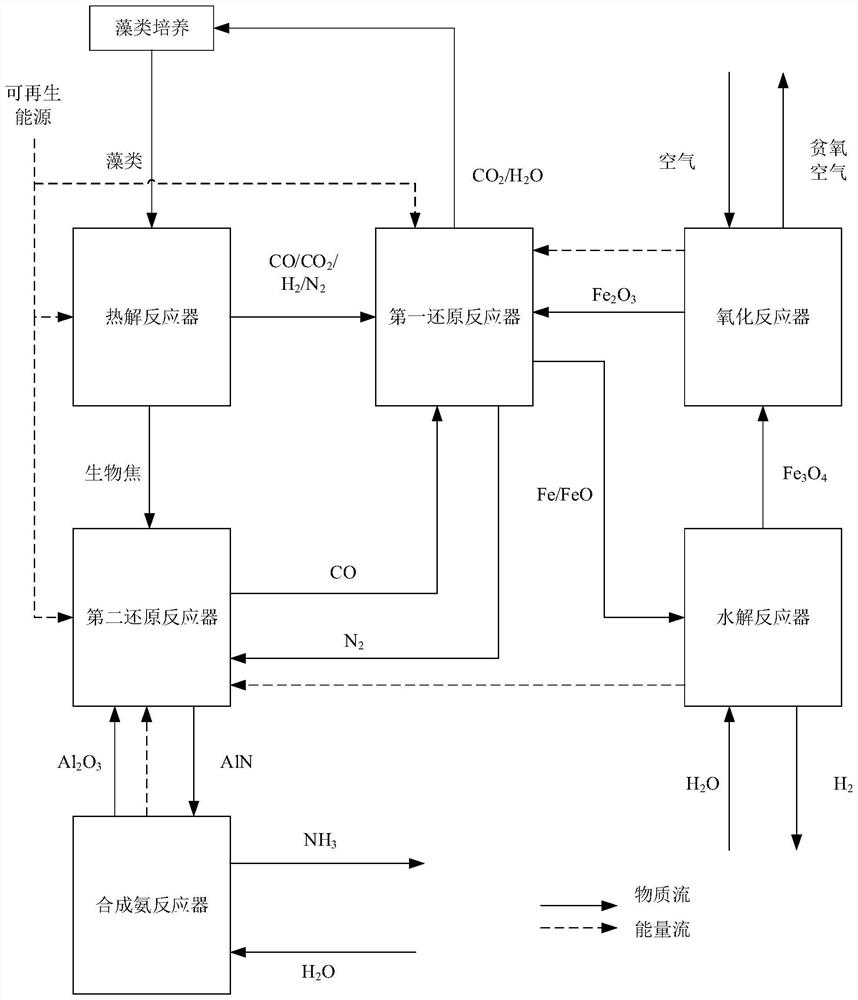
[0038] Such as figure 1 As shown, the present invention provides a kind of chemical chain reaction driven by high nitrogen content biomass to prepare H 2 and NH 3 method, including the following steps:
[0039] Step 1: Pass the biomass with high nitrogen content into the pyrolysis reactor 3, and perform pyrolysis at 600-900°C to produce components of CO, CO 2 , H 2 , N 2 The pyrolysis gas and the biochar whose component is C.
[0040] Step 2: The pyrolysis gas obtained in step 1 is transported into the first reduction reactor 5, and the metal oxygen carrier is reduced to a simple metal or a low-valent metal oxide at 600-1000 ° C to obtain CO 2 , H 2 O and N 2 mixed gas; if the metal oxygen carrier is Fe 2 o 3 , the reaction occurs: CO+Fe 2 o 3 →Fe+CO 2 , H 2 +Fe 2 o 3 →Fe+H 2 O, CO + Fe 2 o 3 →FeO+CO 2 , H 2 +Fe 2 o 3 →FeO+H 2 O.
[0041] Step 3: Pass the metal element or low-valent metal oxide obtained in step 2 into the hydrolysis reactor 6, and mix i...
Embodiment 2
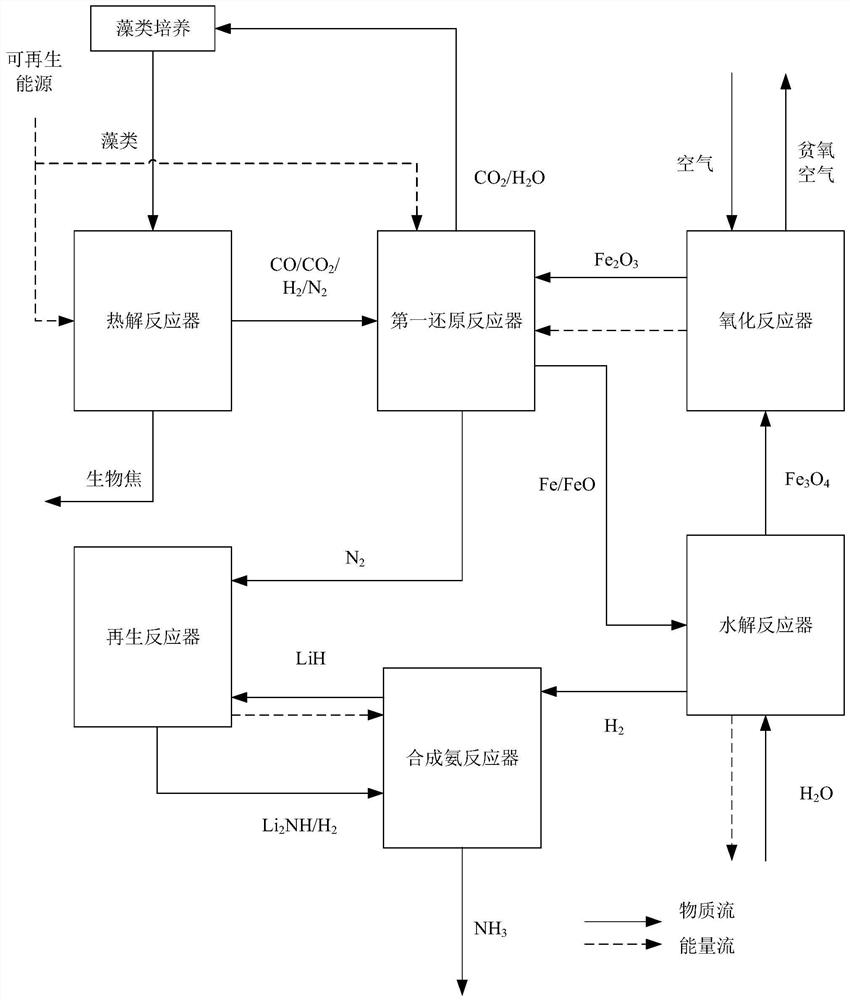
[0047] Such as figure 2 As shown, a chemical chain reaction driven by high nitrogen content biomass to produce biochar and NH 3 method, including the following steps:
[0048] Step 1: Pass the biomass with high nitrogen content into the pyrolysis reactor 3, and perform pyrolysis at 600-900°C to produce components of CO, CO 2 , H 2 , N 2 The pyrolysis gas and the biochar whose component is C.
[0049] Step 2: The pyrolysis gas obtained in step 1 is transported into the first reduction reactor 5, and the metal oxygen carrier is reduced to a simple metal or a low-valent metal oxide at 600-1000 ° C to obtain CO 2 , H 2 O and N 2 mixed gas; if the metal oxygen carrier is Fe 2 o 3 , the reaction occurs: CO+Fe 2 o 3 →Fe+CO 2 , H 2 +Fe 2 o 3 →Fe+H 2 O, CO + Fe 2 o 3 →FeO+CO 2 , H 2 +Fe 2 o 3 →FeO+H 2 O.
[0050] Step 3: Pass the metal element or low-valent metal oxide obtained in step 2 into the hydrolysis reactor 6, and mix it with the incoming H 2 O reacts t...
Embodiment 1 and 2
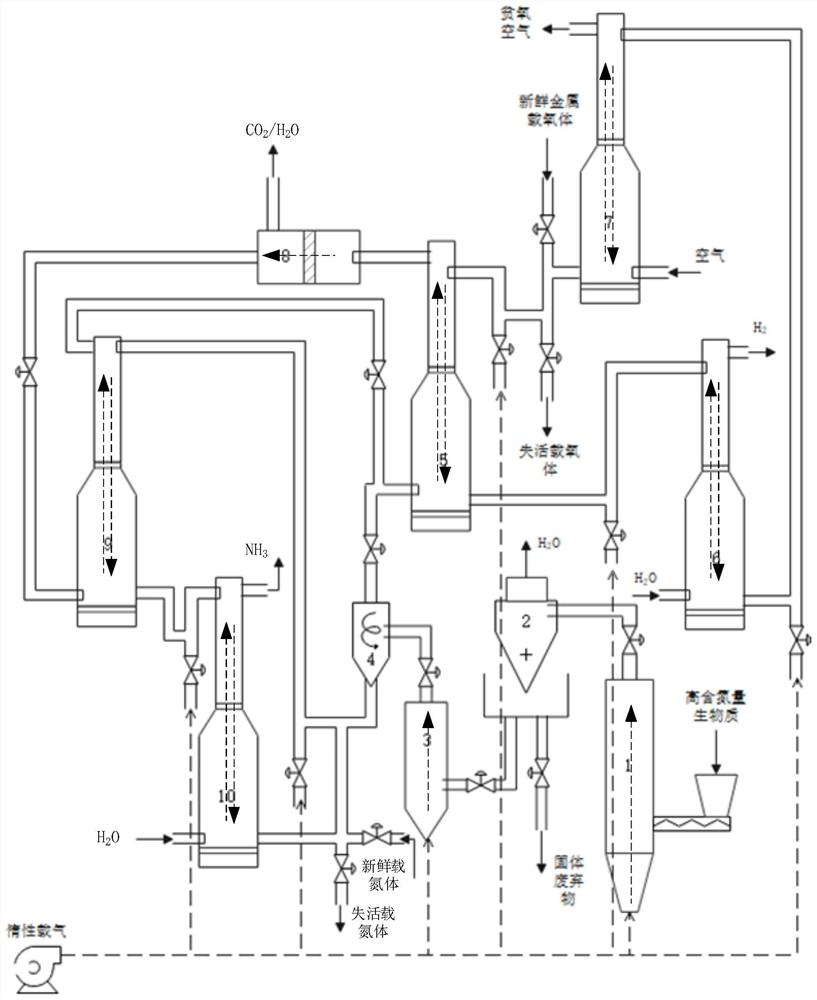
[0056] Biomass with high nitrogen content is one of algae and municipal sludge or a mixture of both.
[0057] Biomass, as a kind of renewable green and clean energy, has received extensive attention from the international community and is an important chemical raw material. However, some special biomasses have high nitrogen content, such as nitrogen-containing algae and municipal sludge, which provide a new direction for the source of nitrogen in the process of ammonia synthesis. As a kind of aquatic biomass, algae has the advantages of fast growth, short cycle, high yield per unit area, strong environmental adaptability, and does not occupy arable land. It has the potential to become a high-quality chemical raw material. The elemental analysis results of some algae show that the nitrogen content can reach about 10%, which makes it a good choice to use algae as a nitrogen source to synthesize ammonia. As a common waste, municipal sludge will not only consume manpower and mate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com