Application of bacteroides fragilis and zwitterionic capsular polysaccharide thereof in preparation of medicine for preventing and treating tumors of genitourinary system
A technology of Bacteroides fragilis and capsular polysaccharide, applied in the field of biomedicine, can solve the problem of no urogenital system cancer prevention and/or treatment, and achieve the effect of enhancing body immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The fermentation culture of embodiment 1 Bacteroides fragilis
[0070] Streak inoculation of Bacteroides fragilis ZY-312 strain on blood plate, anaerobic culture for 48h. Observe the colony morphological characteristics, staining characteristics, size, club shape and distribution, etc.
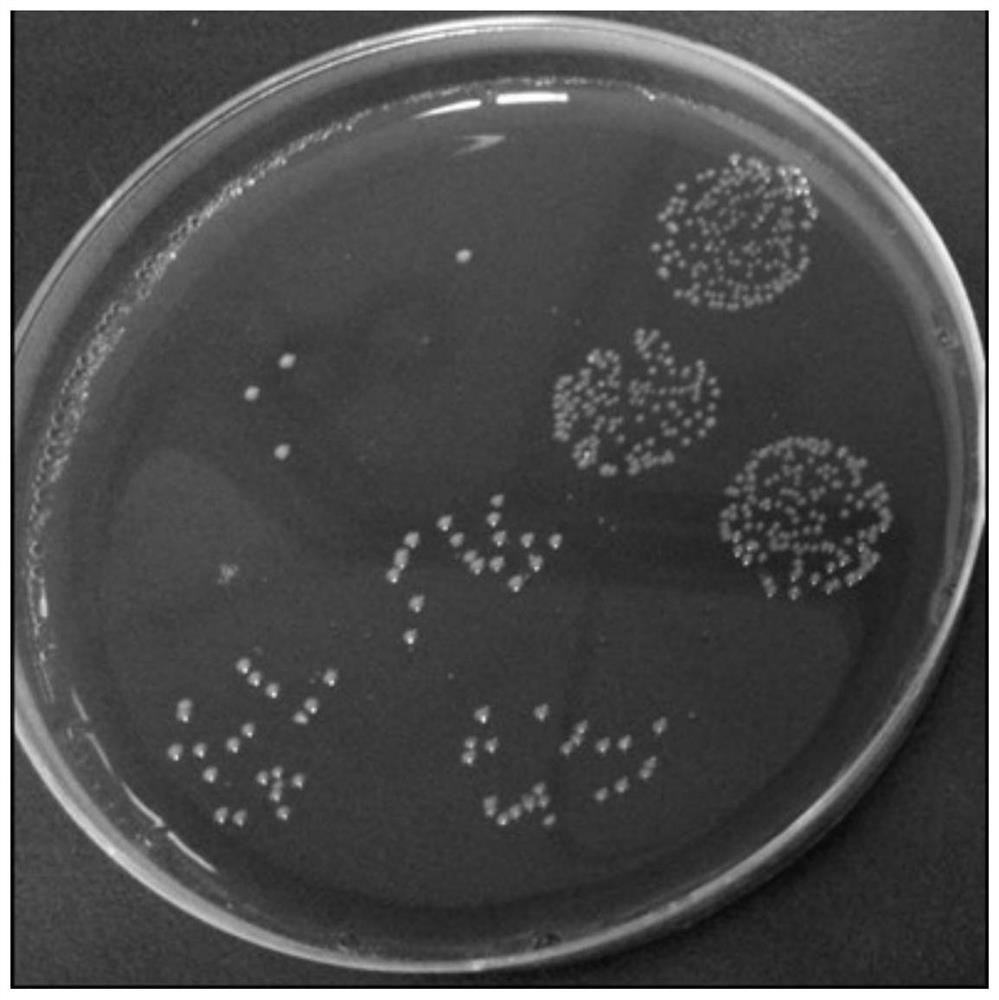
[0071] Colony characteristics: Bacteroides fragilis ZY-312, after being cultured on a blood plate for 48 hours, is slightly convex, translucent, white, smooth, non-hemolytic, and the diameter of the colony is between 1-3mm. See figure 1 .
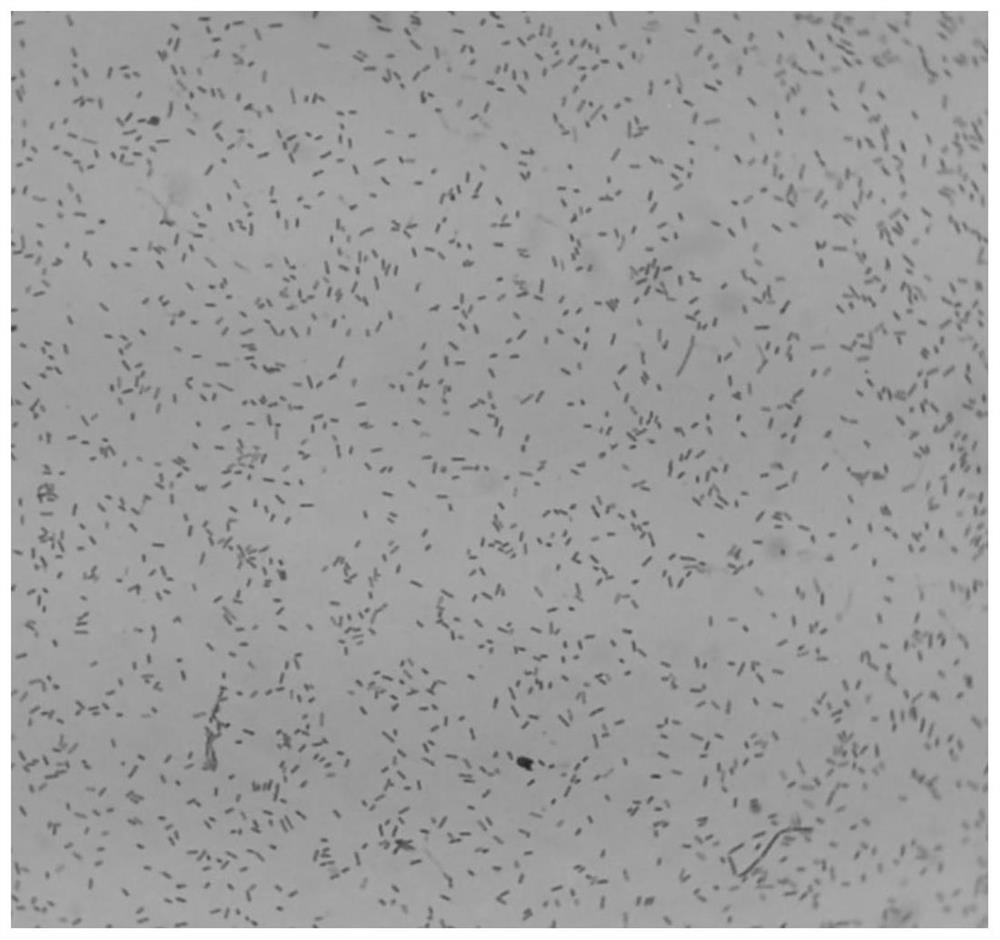
[0072] Morphology under the microscope: Bacteroides fragilis ZY-312 was examined by Gram staining. It is a Gram-negative bacterium with a typical rod shape, blunt rounded ends and dense staining. The uncolored part in the middle of the bacteria is like a vacuole. figure 2 .
[0073] Select a single colony and inoculate it in plant-derived peptone liquid medium for fermentation and culture for 8 hours (at a temperature of 37°C) to obtain a bacteri...
Embodiment 2
[0075] Example 2 Preparation of Bacteroides fragilis capsular polysaccharide
[0076] The bacteria slime prepared in Example 1 was used to carry out the experiment.
[0077] (1) Take 50g of bacteria slime, add 300g of purified water to resuspend the bacteria, adjust its pH to 3.5 with 1mol / L hydrochloric acid solution, extract at 100°C for 1.5h, cool to room temperature, centrifuge at 12000g at room temperature for 10min, take the supernatant to obtain rough sugar solution;
[0078] (2) The crude sugar solution is concentrated by ultrafiltration through a 10KD ultrafiltration membrane to remove small molecular impurities until the conductivity is stable, and the reflux liquid is collected;
[0079] (3) Add an equal volume of 40mmol / L Tris-HCl (pH8.5) to the reflux liquid to convert the salt; .5, containing 0.2mol / L NaCl) gradient elution of 25 column volumes, segmented collection, 100mL / bottle (component), SEC-HPLC follow-up monitoring, combined 206nm absorption peak as a si...
Embodiment 3
[0081] Example 3 Drug efficacy test of Bacteroides fragilis and its zwitterionic capsular polysaccharides on mouse Renca renal carcinoma transplanted tumors
[0082] Renca cells in the logarithmic growth phase were adjusted to 1.5×10 cells with PBS 7 Cells / mL, inoculate 100 μL Renca cell suspension and 100 μL matrigel matrigel solution subcutaneously in the left flank of BALB / c mice with a syringe under sterile conditions. When the transplanted tumor grows to about 40-50mm 3 start administration, divided into control group (normal saline), positive drug group (sunitinib, Pfizer, 50mg / kg), Bacteroides fragilis ZY-312 low (10 6 CFU / only), medium (10 8 CFU / piece), high (10 10 CFU / only) dose group, Bacteroides fragilis ZY-312 inactivated bacteria (10 10 cell / mouse) group, Bacteroides fragilis ZY-312 capsular polysaccharide A (PSA, 500 μg / mouse) was continuously gavaged for 4 weeks. After the last administration, the mice were euthanized, and the transplanted tumors were excise...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com