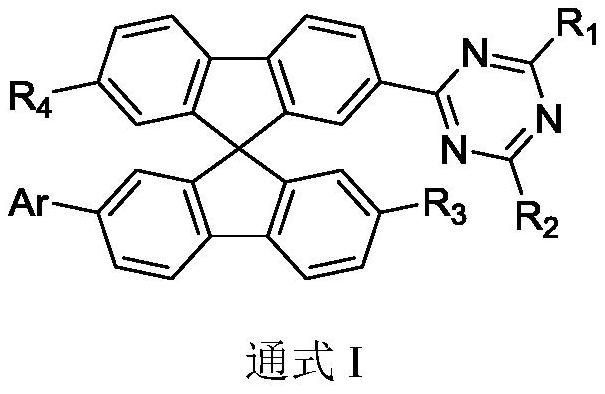
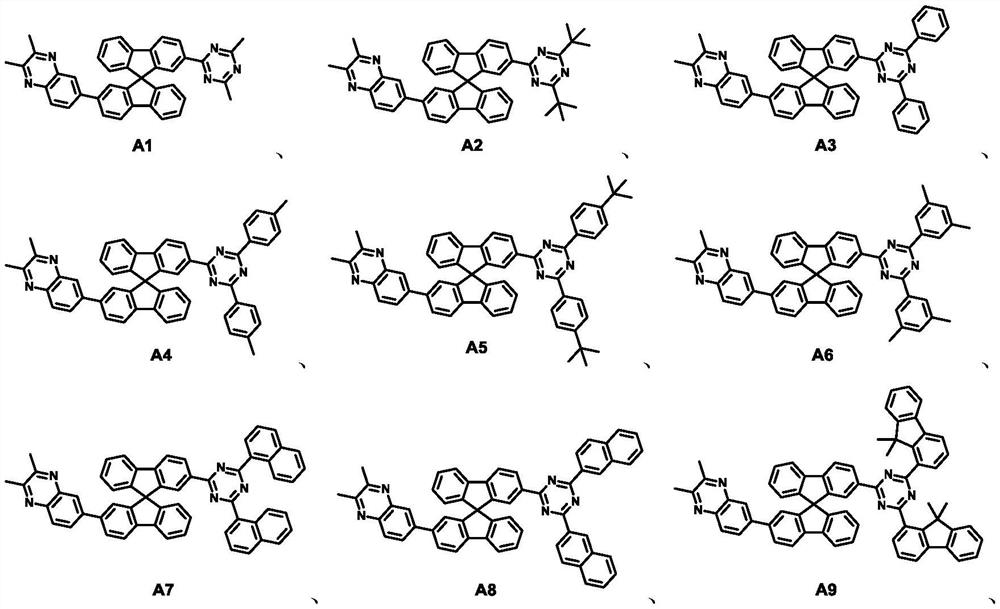
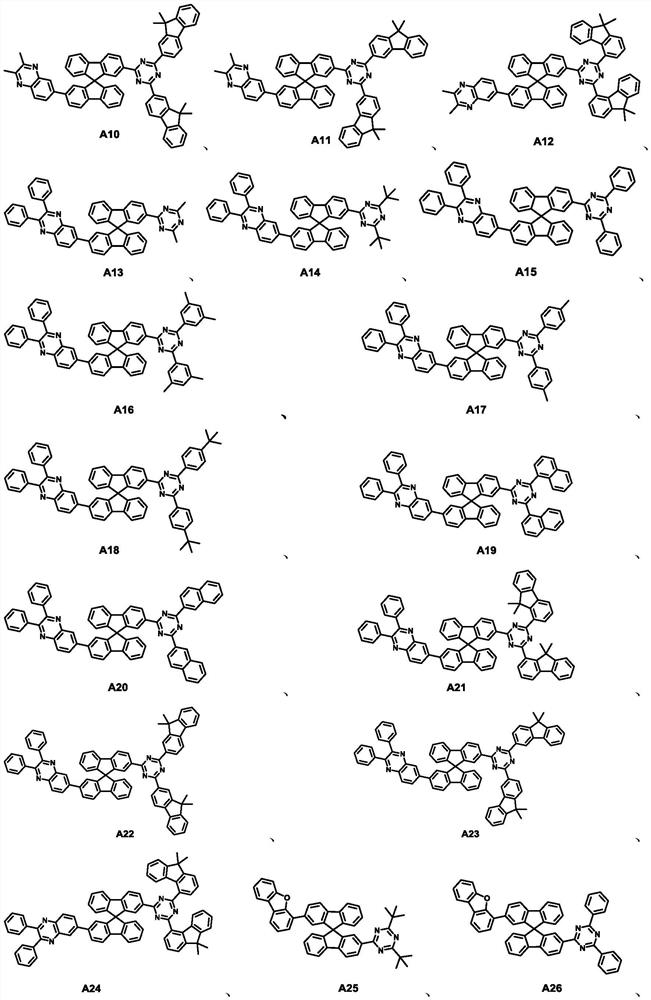
Spirobifluorene compound, electron transport composition and organic electroluminescent device
A technology of electron transport and electron transport layer, which is used in electric solid devices, electrical components, organic chemistry, etc. It can solve the problems of short life and high evaporation temperature, and achieve the effect of prolonging life, reducing evaporation temperature and ensuring stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0049] In a three-necked flask, add 2-bromo-2'-chloro-9,9'-spirobifluorene (30.0g, 69.8mmol), bisboronic acid pinacol ester (18.6g, 73.3mmol) and 1,4-di Hexane (300mL), filled with nitrogen and stirred for 15 minutes, then added potassium acetate (13.8g, 139.6mmol), 1,1'-bis(diphenylphosphine)ferrocenedichloropalladium(II) (1.0g , 1.4mmol), heated to reflux for 2.5 hours. After the reaction was completed, cool to room temperature, filter with silica gel, and evaporate the filtrate to remove the solvent in vacuo to obtain the crude product, which was slurried with n-hexane / toluene (V:V=10:3) for 1 hour, suction filtered, and washed twice with n-hexane to obtain 30.0 g of compound I-1 was off-white solid powder, with a yield of 81% and a purity of 99.02%.
[0050] In a three-necked flask, add intermediate I-1 (30.0g, 62.9mmol), 2-chloro-4,6-diphenyl-1,3,5-triazine (16.8g, 62.9mmol), potassium carbonate ( 26.0g, 157.2mmol), tetrakis-(triphenylphosphine)palladium (1.4g), toluene...
preparation Embodiment 2
[0057] In a three-necked flask, add intermediate I-1 (30.0g, 62.9mmol), 2-chloro-4,6-bis(9,9'-dimethylfluorene)-1,3,5-triazine (31.5 g, 62.9 mmol), potassium carbonate (26.0 g, 157.2 mmol), tetrakis-(triphenylphosphine) palladium (1.4 g), toluene (250 mL), ethanol (50 mL) and water (80 mL), heated under nitrogen Reflux for 10 hours. After the reaction was completed, cool to room temperature, extract with toluene and water, take the organic layer and filter it with silica gel, evaporate the filtrate to remove the solvent in vacuo to obtain a crude product, which was recrystallized with a mixed solvent of dichloromethane / ethanol to obtain 46 g of compound I as a white solid powder -4, the yield was 90%, and the purity was 99.7%.
[0058] In a three-necked flask, add intermediate I-4 (42.0g, 51.6mmol), pinacol diboronate (15.7g, 61.9mmol) and toluene (300mL), fill with nitrogen and stir for 15 minutes, then add potassium acetate (10.1 g, 103.2 mmol), tris(dibenzylidene indeneac...
preparation Embodiment 3
[0064]In a three-necked flask, add intermediate I-1 (30.0g, 62.9mmol), 2-chloro-4,6-bis(1-naphthyl)-1,3,5-triazine (23.14g, 62.9mmol) , potassium carbonate (26.0g, 157.2mmol), tetrakis-(triphenylphosphine)palladium (1.4g), toluene (250mL), ethanol (50mL) and water (80mL), heated to reflux for 10 hours under nitrogen protection. After the reaction is completed, cool to room temperature, extract with toluene and water, take the organic layer and filter it with silica gel, evaporate the filtrate to remove the solvent in vacuo to obtain a crude product, which is recrystallized with a mixed solvent of dichloromethane / ethanol to obtain 40 g of compound I as a white solid powder -6 with a yield of 93% and a purity of 99.2%.
[0065] In a three-necked flask, add intermediate I-6 (35.0g, 51.6mmol), pinacol diborate (15.7g, 61.9mmol) and toluene (500mL), fill with nitrogen and stir for 15 minutes, then add potassium acetate (10.1 g, 103.2 mmol), tris(dibenzylidene indeneacetone) dipall...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com