Preparation method and application of mono-and bis-phenylthioimine vanadium olefin polymerization catalyst
A technology of bisphenylthiimide vanadium olefin and polymerization catalyst, which is applied in the field of preparation of olefin polymerization catalyst, can solve the problems of poor copolymerization effect and high temperature resistance, and achieves the effect of simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
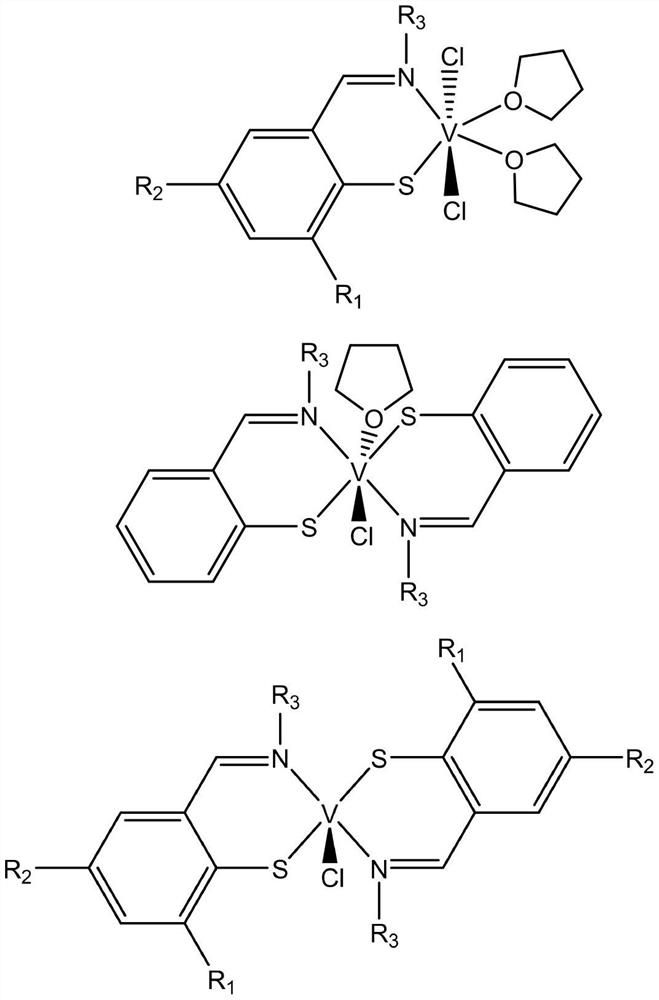
[0038] Embodiment 1: the synthesis of monophenylsulfimide vanadium metal complex
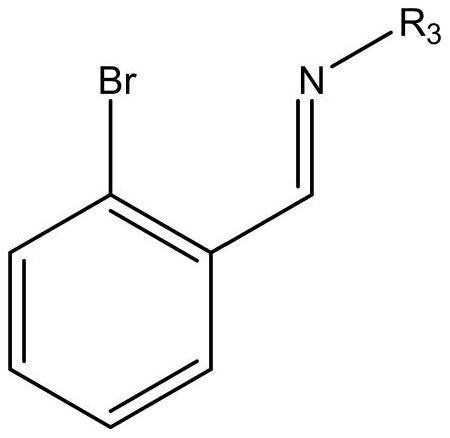
[0039] In a clean reactor, o-bromobenzaldehyde or benzaldehyde derivatives (30.00mmol) and an equivalent of organic aniline (30.00mmol) were dissolved in 100mL of methanol solution, and 4-5 drops of formic acid were added dropwise and reacted for 12 hours to obtain The yellow solution was removed from the solvent to obtain a yellow oily liquid or solid (yield 86-92%). The oily liquid uses molecular sieve to remove water for later use.
[0040] The above-mentioned ligand 1 (1.00 mmol) was weighed and added into a Schlenk bottle, and 20 mL of toluene was transferred in a nitrogen atmosphere. Place the Schlenk bottle in a low-temperature bath at –78°C, and use a syringe to accurately measure n BuLi (0.66mL, 1.05mmol) was slowly added dropwise into the Schlenk bottle and kept warm for 1 hour. Add elemental S (0.03g, 1.00mmol) into the reaction vial using an ampoule, stir at low temperature for 1 ...
Embodiment 2
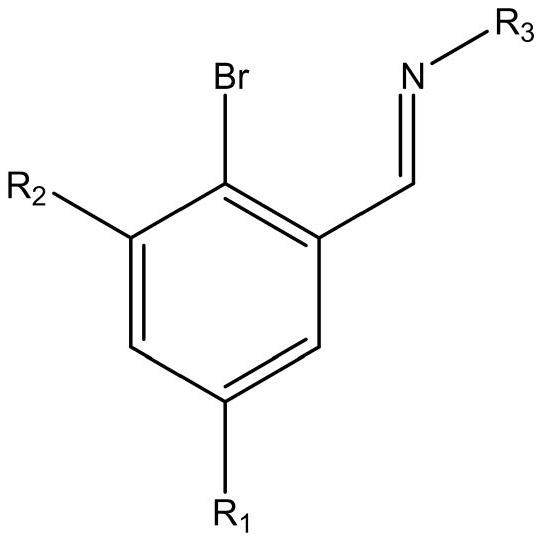
[0042] The experimental operation is the same as in Example 1, using 2-bromo-3,5-disubstituted benzaldehyde or benzaldehyde derivatives (6.00mmol), organic aniline (6.00mmol), to obtain 2-bromo-3,5-disubstituted Imine ligand yellow solid product (yield: 75-87%).
[0043] The above-mentioned ligand 2 (1.00 mmol) was weighed and added into a Schlenk bottle, and 20 mL of ether was transferred in a nitrogen atmosphere. Place the Schlenk bottle in a low-temperature bath at –78°C, and use a syringe to accurately measure nBuLi (0.66mL, 1.05mmol) was slowly added dropwise into the Schlenk bottle, stirred at low temperature for 1 hour and then at room temperature for 3 hours until the solution turned red. Simple S (0.03 g, 1.00 mmol) was added into the reaction vial using an ampoule, and stirred overnight at room temperature. At –78°C, the resulting yellow solution was added dropwise to 10 mL of diethyl ether in VCl 3 (THF) 2 (0.37g, 1.00mmol) solution, then slowly rise to room tem...
Embodiment 3
[0047] Embodiment 3: the synthesis of bisphenylsulfimide vanadium metal complex
[0048] The experimental operation is the same as in Example 1; using ligand 1 (1.00mmol), n BuLi (0.66mL, 1.05mmol), S (0.03g, 1.00mmol), VCl 3 (THF) 2 (0.19g, 0.50mmol), the bisphenylsulfimide vanadium product was obtained as a brown solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com