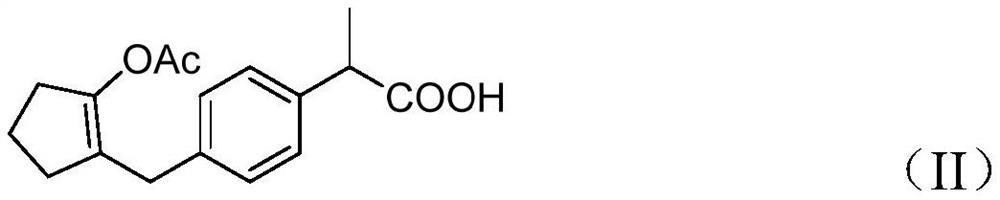Preparation method of loxoprofen sodium degradation impurity
A technology for loxoprofen sodium and impurities is applied in the field of preparation of loxoprofen sodium oxidative degradation impurities, can solve problems such as no reports on the preparation of impurities, and achieve the effects of less side reactions, simple operation and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
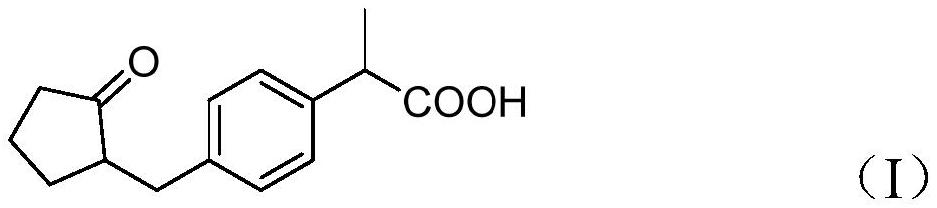
[0048] Put 13g of 2-(4-((2-cyclopentanonyl)methyl)phenyl)propionic acid (compound I), 16.35g of acetic anhydride, and 150ml of tetrahydrofuran into a 250ml three-necked flask, stir and add 2mL of concentrated sulfuric acid, 10~ React at 20°C for 4 hours and monitor the reaction by sampling TLC (ethyl acetate:n-hexane=2:1). After the reaction of the raw materials was complete, sodium bicarbonate solution was added to adjust the pH to 5-6, and the layers were separated. The organic phase was concentrated under reduced pressure until there was no distillate, and 14.3 g of the concentrate was obtained. The reaction was directly carried out to the next step without purification.
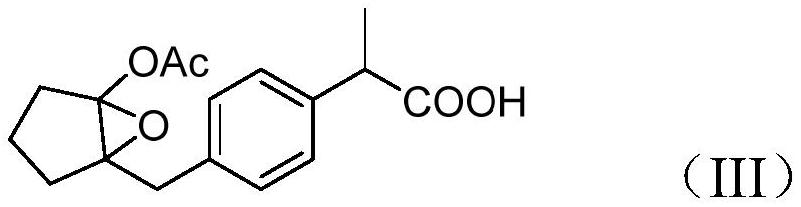
[0049] Put 14g of the concentrate from the previous step and 200ml of dichloromethane into a 500ml three-necked bottle, add 44g of 50% hydrogen peroxide dropwise at a temperature of 20-30°C, and react for 20 hours at 20-30°C after dropping. Sample TLC (ethyl acetate:n-hexane=2:1) was used to monitor the...
Embodiment 2
[0053] Put 13g of 2-(4-((2-cyclopentanonyl)methyl)phenyl)propionic acid (compound I), 16.35g of acetic anhydride, and 150mL of dioxane into a 250ml three-necked flask, stir and add 3mL of hydrochloric acid, React at 30-40°C for 6 hours, and monitor the reaction by sampling TLC (ethyl acetate:n-hexane=2:1). After the reaction of the raw materials was complete, sodium bicarbonate solution was added to adjust the pH to 5-6, and the layers were separated. The organic phase was concentrated under reduced pressure until there was no distillate, and 15.4 g of the concentrate was obtained. The reaction was directly carried out to the next step without purification.
[0054] Put 15g of the concentrate from the previous step and 200ml of dichloromethane into a 500ml three-necked bottle, add 10g of m-chloroperoxybenzoic acid at a temperature of 20-30°C, and react for 10 hours at 20-30°C. Sample TLC (ethyl acetate:n-hexane=2:1) was used to monitor the reaction. After the raw material r...
Embodiment 3
[0057] Put 13g of 2-(4-((2-cyclopentanonyl)methyl)phenyl)propionic acid (Compound I), 16.35g of acetic anhydride, 50ml of dichloromethane into a 250mL reaction flask, stir and add 3mL of perchloric acid , react at 10-20° C. for 2 hours and monitor the reaction by sampling TLC (ethyl acetate:n-hexane=2:1). After the reaction of the raw materials was complete, sodium bicarbonate solution was added to adjust the pH to 5-6, and the layers were separated. The organic phase was concentrated under reduced pressure until there was no distillate, and 16.3 g of the concentrate was obtained. The reaction was directly carried out to the next step without purification.
[0058] Put 16g of the concentrate from the previous step and 200ml of toluene into a 500mL reaction bottle, add 44g of peracetic acid dropwise at a temperature of 20-30°C, and react for 10 hours at 20-30°C after dropping. Sample TLC (ethyl acetate:n-hexane=2:1) was used to monitor the reaction. After the reaction of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com