Alkaloid with antitumor activity as well as preparation method and application thereof
An anti-tumor activity, alkaloid technology, applied in anti-tumor drugs, organic active ingredients, organic chemical methods, etc., can solve the problem of no cytotoxicity, achieve high industrial application prospects, good biocompatibility, and yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
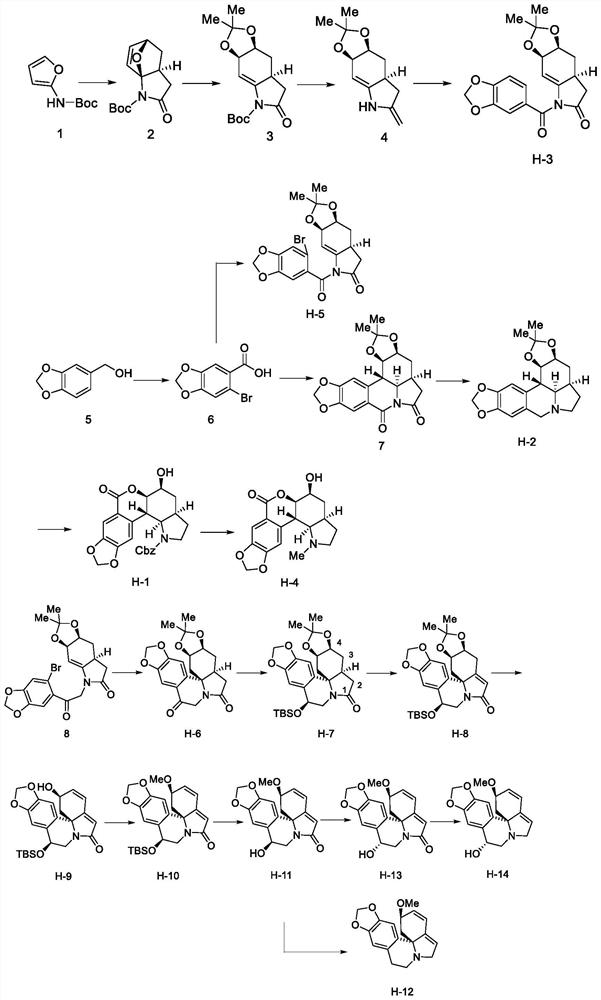
[0057] The preparation of each compound specifically comprises the following steps:
[0058] Synthesis of compound 2
[0059] Compound 1 (N-tert-butoxycarbonyl-N-methylethylenediamine) (500 mg) was dissolved in redistilled THF (tetrahydrofuran) (12 mL), and n-butyl lithium (2.4M in THF, 1.05mL), stirred at -50°C for 30min to obtain solution A; dissolved crotonic acid (258.27mg) in redistilled THF (5mL), and added N-methylmorpholine (0.325mL) at -10°C, Then slowly add isobutyl chloroformate (0.375mL) and stir at -10°C for 15min, the reaction solution becomes white and turbid. Slowly add solution A to solution B at -50°C, stir at -50°C for 30min, then stop the reaction, add saturated ammonium chloride to quench, and extract the aqueous phase with EA (ethyl acetate) (10mL×3), The organic phases were combined, washed with saturated brine (10 mL), dried with anhydrous sodium sulfate, filtered, evaporated under reduced pressure and spin-dried to obtain a yellow oil, which was left...
Embodiment 2
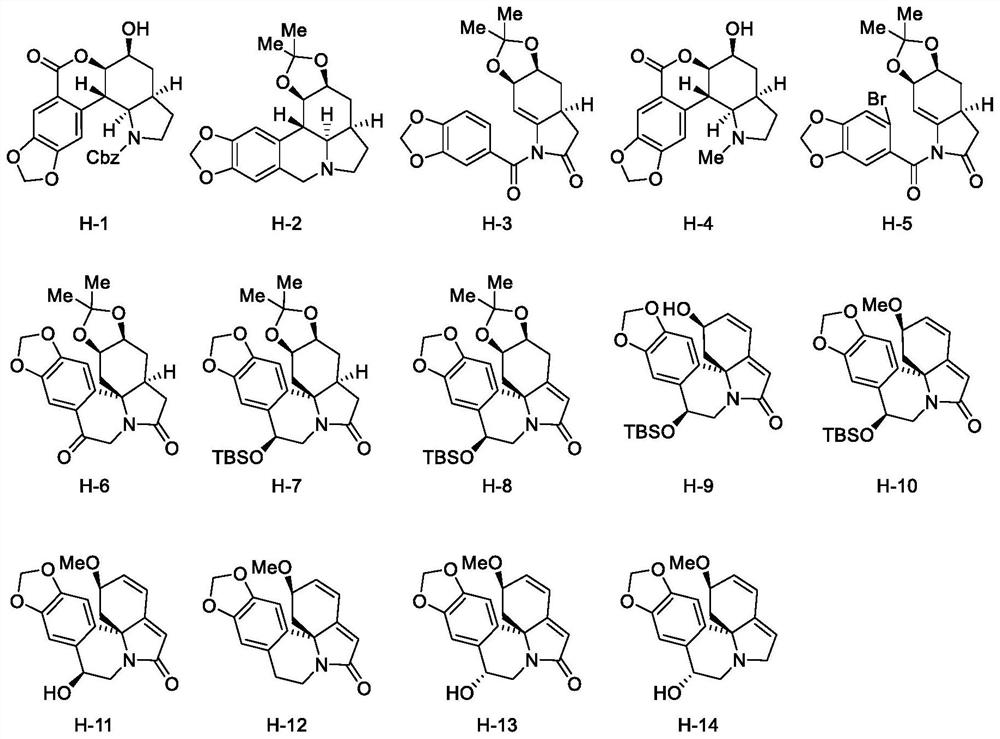
[0096] Example 2 Product H-1 to H-6 antitumor activity test
[0097] The products H-1 to H-6 prepared in Example 1 were tested for antitumor activity, and the specific steps are as follows:
[0098] The growth inhibitory activity of various tumor cells was screened by MTT method, and doxorubicin was used as a positive control drug at the same time. Digest the cells in the logarithmic growth phase with 0.25% trypsin, prepare a single-cell suspension, and inoculate 3000-4000 cells / well in a 96-well plate according to the difference in cell growth rate, and add 100 μL of cell suspension to each well . After 24h, add the concentration of 10μmol L -1 Add 100 μL (final concentration of DMSO 50 .
[0099] The growth inhibitory activity of various cancer cells was screened by MTT method, and adriamycin was used as a positive control drug. Table 1 shows the effects of products H-1 to H-6 on prostate cancer cells LNcaP, human cervical squamous cell carcinoma cells SiHa, human The s...
Embodiment 3
[0103] Example 3 Product H-3 Antitumor Activity Test
[0104] According to the method in Example 2, alkaloid H-3 was tested for prostate cancer cell LNcaP, human breast cancer cell MCF-7, human cervical squamous cell carcinoma cell SiHa, and human prostate cancer cell PC-3 at different concentrations. inhibition.
[0105] Table 2 shows the survival rate of alkaloid H-3 to LNcaP, MCF-7, SiHa, PC-3 at different concentrations detected by MTT method.
[0106] Table 2 The survival rate of alkaloid H-3 to LNcaP, MCF-7, SiHa, PC-3 at different concentrations detected by MTT method
[0107]
[0108] It can be seen from the data in Table 2 that the concentration of alkaloid H-3 at 10 μmol L -1 The concentration has a certain inhibitory rate on LNcaP, MCF-7, SiHa, PC-3 cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com