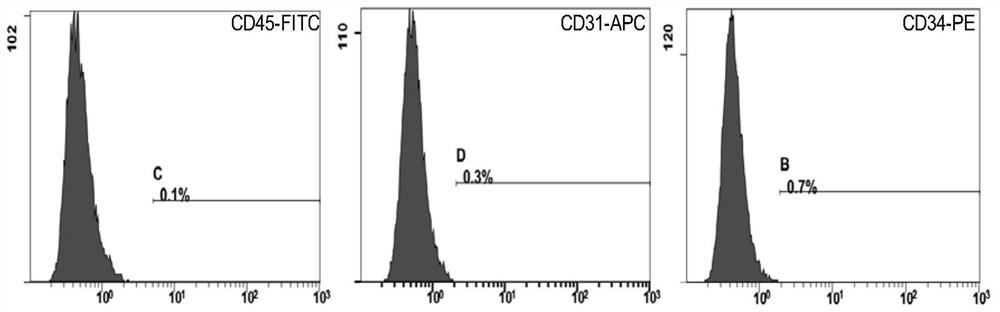
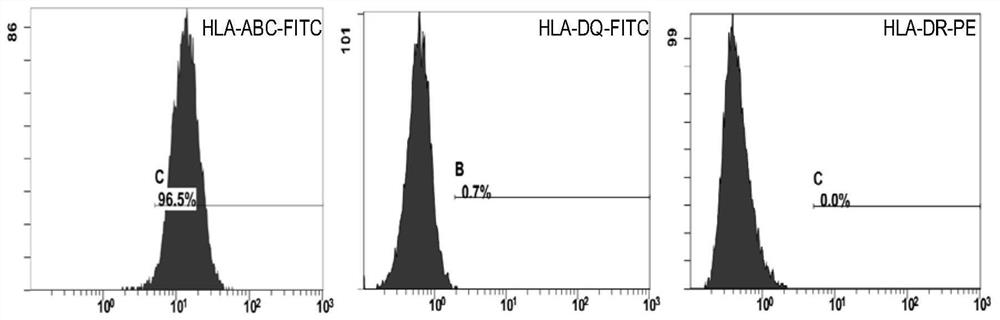
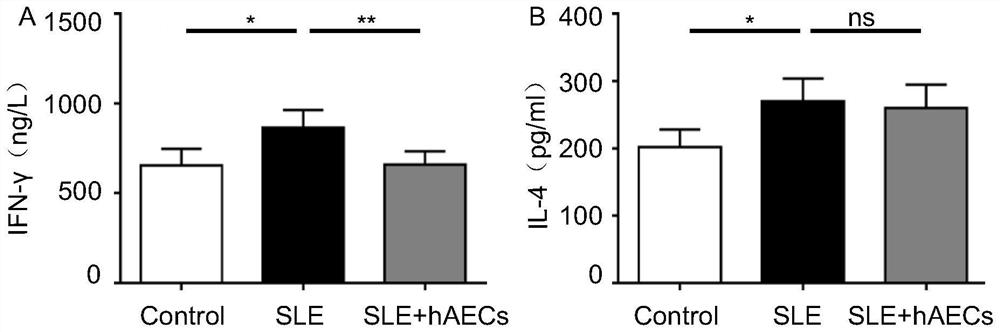
Therapeutic application of human amniotic epithelial cells in autoimmune diseases
A technology of amniotic membrane epithelial cells and autoimmunity, applied in allergic diseases, artificial cell constructs, cell culture active agents, etc., can solve the problems of side effects, poor treatment effect, etc., to avoid immune rejection, reduce sources, Effects from a wide range of sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0075] Example 1-1 Construction of an experimental autoimmune thyroiditis model
[0076] 1. Purchase and feeding of experimental animals
[0077] CBA / J mice, SPF grade, 30-40g, female, were provided by Shanghai Southern Model Animal Center. Raise in separate cages. The same group of mice were placed in the same cage. Six mice per cage were raised in the Experimental Animal Center of Zhejiang University. Food and water were freely available.
[0078] 2. Experimental drugs
[0079] (1) Complete Freund's adjuvant (complete Freund's adjuvant, CFA) American Sigma Company
[0080] (2) Incomplete Freund's adjuvant (IFA) Sigma, USA
[0081] (3) Porcine thyroglobulin (pTg) Sigma, USA
[0082] 3. Grouping of experimental animals
[0083] Grouped according to different treatment times (Table 1), each group was repeated at least three times, and each experiment consisted of 9 rats in each group, including 3 rats in the model treatment group (EAT+hAECs group), 3 rats in the simple mo...
Embodiment 1-2
[0090] The preparation of embodiment 1-2 human amnion cell experiment solution
[0091] 1. Preparation of amnion epithelial cell culture medium: add 56ml KSR to 500ml DMEM / F12; 6ml L-Glutamine; 6ml Sodium Pyruvate; 6ml MEMNEAA; 600μl 2-ME; 2000× EGF and 100× P / S Add to;
[0092] 2. Preparation of 2000×EGF: Add 1ml of sterile ddH2O to the EGF packaging tube, let it stand for 5-10min to dissolve, then add 4ml of diluent (5% Trehalose PBS), mix well and then distribute to 1.5ml EP tube 100μl per tube;
[0093] 3. Preparation of digestion termination solution: DMEM / F12+10% FBS;
[0094] 4. Preparation of cryopreservation solution: 40% FBS + 50% culture medium + 10% DMSO.
Embodiment 1-3
[0095] Example 1-3 Isolation of human amniotic epithelial cells
[0096] 1. The source of human amniotic membrane
[0097] After the mother’s authorization and consent, the placental tissue of the healthy parturient (HIV, syphilis, hepatitis A, hepatitis B, hepatitis C and other serological reactions were all negative) after cesarean section was taken, the placenta was cut with a cross knife, and the whole amniotic membrane was obtained by mechanical separation.
[0098] 2. Isolation of hAECs
[0099] Wash the amniotic membrane three times with the sterile PBS solution that has added double antibody (P / S), wash away blood and other impurities, and transfer the amniotic membrane to a 50ml centrifuge tube.
[0100] Add 10ml of 0.25% trypsin (bathed at 37°C in advance) to digest for 30s, invert 20 times, and transfer the amnion into another 50ml centrifuge tube.
[0101] Add 15ml of 0.25% trypsin (bathed at 37°C in advance) to the centrifuge tube, and after digestion in a water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com