Fluorescent substrate for detecting human cytochrome P450 3A4 as well as preparation method and application of fluorescent substrate
A cytochrome and fluorescent substrate technology, applied in the field of medicine, can solve problems such as complex methods, and achieve the effects of high detection throughput, efficient and rapid real-time detection, and high-throughput detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
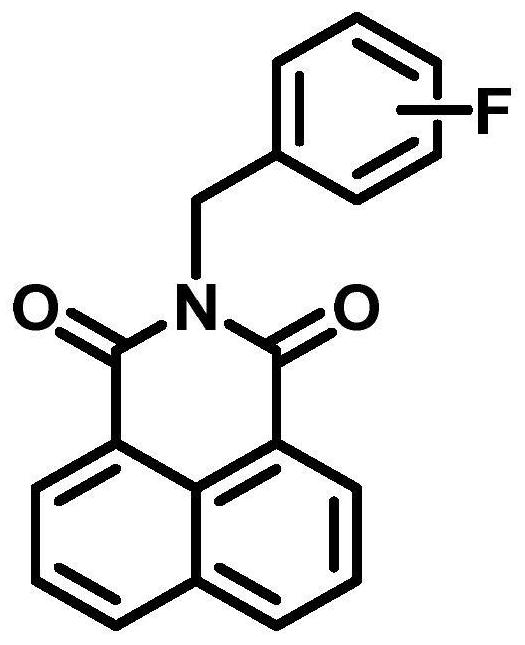
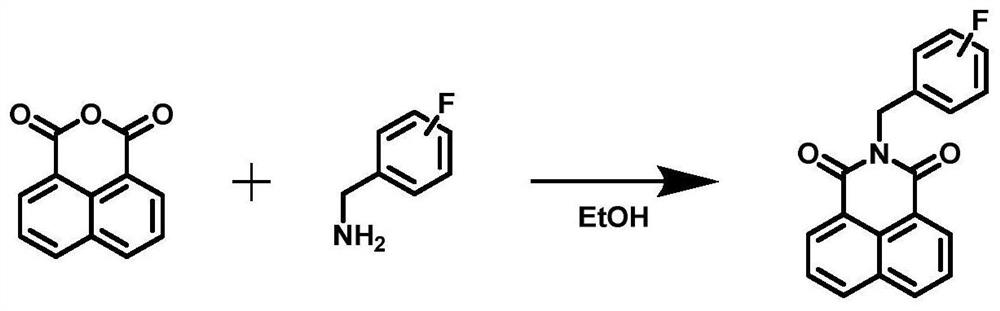
[0037] Embodiment 1 The chemical synthesis of N-fluorobenzyl-1,8-naphthalimide compounds
[0038] (1) Dissolve one equivalent of 1,8-naphthalene dicarboxylic anhydride in 20 mL of absolute ethanol, add 1.2 equivalents of 2-fluorobenzylamine / 3-fluorobenzylamine / 4-fluorobenzylamine, stir and slowly heat to Reflux, and continue for 3h;
[0039] (2) The reaction solution was naturally cooled to room temperature, filtered to obtain a white filter cake as a crude product, and purified by silica gel chromatography (eluent: dichloromethane) to obtain pure N-(2'-fluoro Benzyl)-1,8-naphthalimide (B-2, yield: 89.8%, see figure 2 a).
[0040] 1 H NMR (600MHz, CDCl 3 )δ8.63(d, J=7.3Hz, 2H), 8.23(d, J=8.2Hz, 2H), 7.77(t, J=7.7Hz, 2H), 7.30(t, J=7.5Hz, 1H) ,7.24–7.18(m,1H),7.10–6.99(m,2H),5.48(s, 2H). 13 C NMR (150MHz, CDCl 3 )δ 164.13, 161.60, 159.96, 134.17, 131.66, 131.58, 129.25, 129.22, 128.92, 128.86, 128.29, 127.01, 124.19, 124.09, 124.07, 124.05, 122.534, 137.5
[0041] Obtain...
Embodiment 2
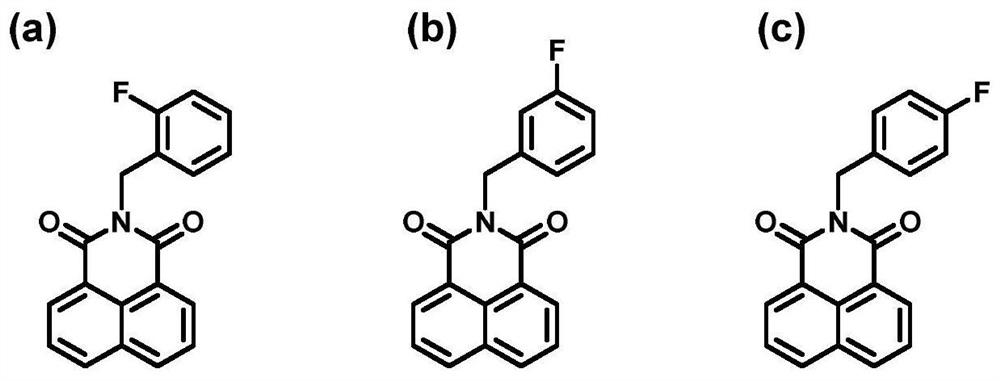
[0045] Example 2 Metabolic phenotype analysis of the specificity of N-fluorobenzyl-1,8-naphthalimide substrates
[0046] (1) Prepare 180 μl phase I metabolic reaction system, including PBS buffer (100 mM) at pH 7.4, commercial human P450 enzyme (10 nM), B-1 (1 μM), B-2 (1 μM), B-3 ( 1 μM) at 37°C for 3 minutes with shaking;
[0047] (2) Add 20 μl of NADPH with a final concentration of 1 mM to the reaction system to initiate the reaction;
[0048] (3) After 30 minutes, add 100 μl of glacial acetonitrile, shake vigorously, terminate the reaction, centrifuge at 20,000 g for 30 minutes, and take the supernatant for testing;
[0049] (4) Detection of the fluorescent signal of the product (E x =450nm,E m =555nm); Obtain the fluorescence intensity of the product in each sample, B-2, B-3, the subtype selectivity of the same family of B-1 are respectively 3.6,7.9,72.6 times ( Figure 4 a, 4b, 4c).
[0050] Conclusion: Among the three fluorescent substrates, the B-1 product was for...
Embodiment 3
[0051] The enzymatic kinetic analysis of embodiment 3 substrate B-1
[0052] (1) Prepare 180 μl phase I metabolic reaction system, including PBS buffer (100mM) at pH 7.4, commercialized human CYP 3A4 (0.025nM) / commercialized human liver microsomes (0.005mg / ml), different concentrations of B -1 Pre-incubate with shaking at 37°C for 3 minutes;
[0053] (2) Add 20 μl of NADPH with a final concentration of 1 mM to the reaction system to initiate the reaction;
[0054] (3) After 20 minutes, add 100 μl of glacial acetonitrile, shake vigorously, terminate the reaction, centrifuge at 20,000 g for 30 minutes, and take the supernatant for testing;
[0055] (4) Detection of the fluorescent signal of the product (E x =450nm,E m =555nm); the fluorescence intensity of the product in each sample was obtained, and the kinetic curve of the enzymatic reaction was fitted. In both single enzyme and commercial human liver microsomes, Mie kinetics were exhibited, and K m Very close, respective...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com