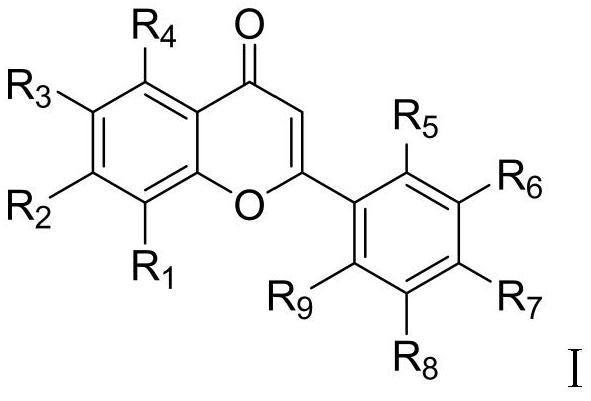
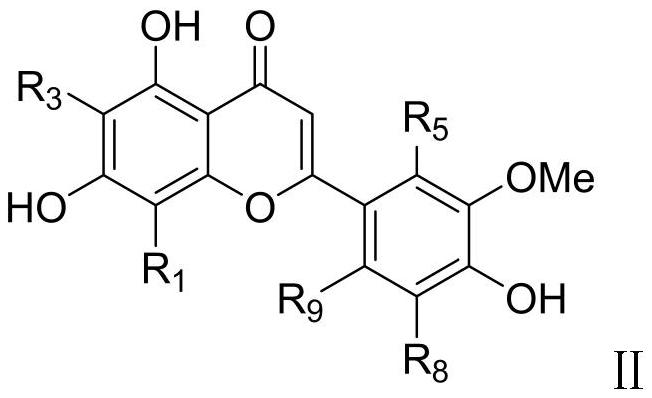
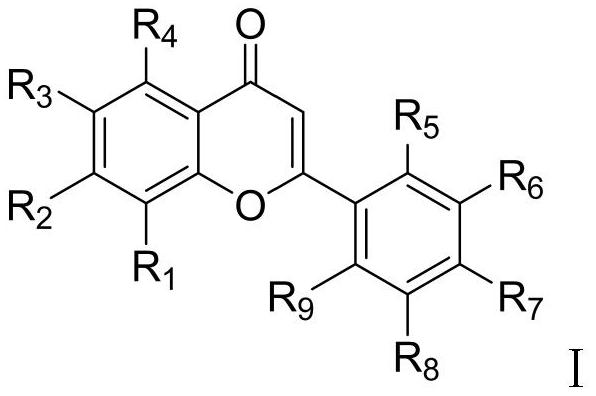
Preparation method of ephedrone compound
A technology for cannabinoids and compounds, applied in the field of chemical synthesis, can solve the problems of flavonoids having no psychoactive properties, being difficult to amplify, and having long synthesis steps, and achieving the effects of low synthesis cost, short production period and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、4
[0117] The synthesis of embodiment 1,4'-hydroxyl-3'-methoxybenzoyl ethyl acetate
[0118]
[0119] Add 60% NaH (6.00 g, 150 mmol) to the reaction flask, under nitrogen protection, add toluene (60 mL) and DEC (11.82 g, 100 mmol), stir, and heat to reflux. A solution of 4'-hydroxy-3'-methoxyacetophenone (12.82 g, 50 mmol) in toluene (60 mL) was added dropwise. Reflux for 4 hours after addition. Cool down to room temperature, adjust the pH to neutral with acetic acid, add saturated ammonium chloride aqueous solution (150mL), extract the aqueous phase with ethyl acetate (100mL×3), combine the organic phases with saturated ammonium chloride aqueous solution (100mL×2 ), washed with saturated brine (100mL×2). The organic phase was dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated to dryness, then added with n-hexane (50 mL) and stirred overnight. Filtration gave ethyl 4'-hydroxy-3'-methoxybenzoylacetate (15.43 g, 93.9%) as an orange-y...
Embodiment 2
[0120] Embodiment 2, the synthesis of 2-isopentenyl-1,3,5-trihydroxybenzene
[0121]
[0122] Add water (80 mL) and potassium hydroxide (4.87 g, 73.92 mmol) into the reaction flask, and stir. 1,3,5-Trihydroxybenzene (10.0 g, 79.20 mmol) was added. Cool down in an ice-water bath, add bromoisoamyl (6.6 g, 41.2 mmol) dropwise, and stir overnight. Ethyl acetate (100 mL) was added, and the pH was adjusted to 5.0 with 1N hydrochloric acid. The aqueous phase was extracted with ethyl acetate (50 mL×3), the organic phases were combined, washed with saturated brine 100 mL×2, and dried over anhydrous sodium sulfate. Suction filtration, the filtrate was concentrated to dryness, the resulting oil was separated by column chromatography (silica gel 200-300 mesh, PE:EA=8:1→2:1), and the orange oil 2-prenyl-1,3 , Synthesis of 5-trihydroxybenzene (3.17 g, 20.6%).
Embodiment 3
[0123] Embodiment 3, the synthesis of cannabinoid B and / or cannabinoid B isomer
[0124]
[0125] Add 4'-hydroxy-3'-methoxybenzoyl ethyl acetate (18.0g, 75.6mmol) and 2-isopentenyl-1,3,5-trihydroxybenzene (9.8g, 50.5 mmol), stir. Under nitrogen protection, heat to 200°C and maintain the temperature for 3 hours. Down to room temperature, the resulting product was subjected to repeated normal-phase silica gel column chromatography (silica gel 200-300 mesh, PE:EA=10:1→2:1) and then repeated reverse-phase silica gel column chromatography (reversed-phase silica gel 200 -300 mesh, water:methanol=80:20→30:70), and recrystallized by methanol to obtain about 1g of orange-yellow solid cannabinoid B (yield about 5.4%), and simultaneously obtain orange-yellow solid cannabinoid B isomer About 1g (yield about 5.4%).
[0126] Cannabinoid B: MS (ESI): 369.1 [M+H] + , 1 H NMR (400MHz, DMSO-d 6 ),δ:13.22(s,1H),10.83(s,1H),9.95(s,1H),7.56(d,1H,),7.54(s,1H),6.92(d,1H),6.89(s ,1H), 6.55(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com