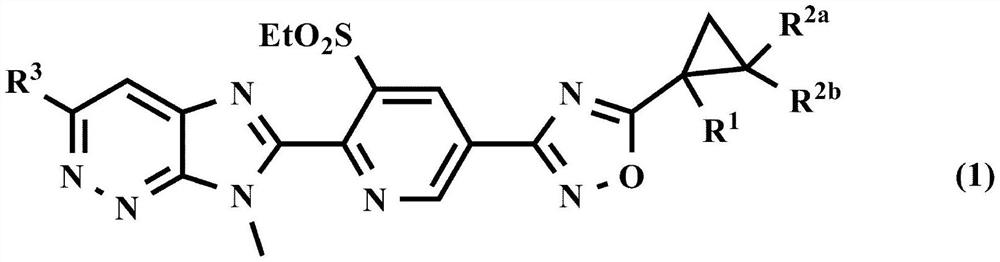
Agricultural and horticultural insecticide or external or internal parasite control agent for animals, containing imidazopyridazine compound having substituted cyclopropane oxadiazole group or salt thereof as active ingredient, and method for using same
A technology of cyclopropane oxadiazolyl and imidazopyridazine, which is applied in the field of external or internal parasite control agents, and can solve the problems of unspecified disclosure of imidazopyridazine compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
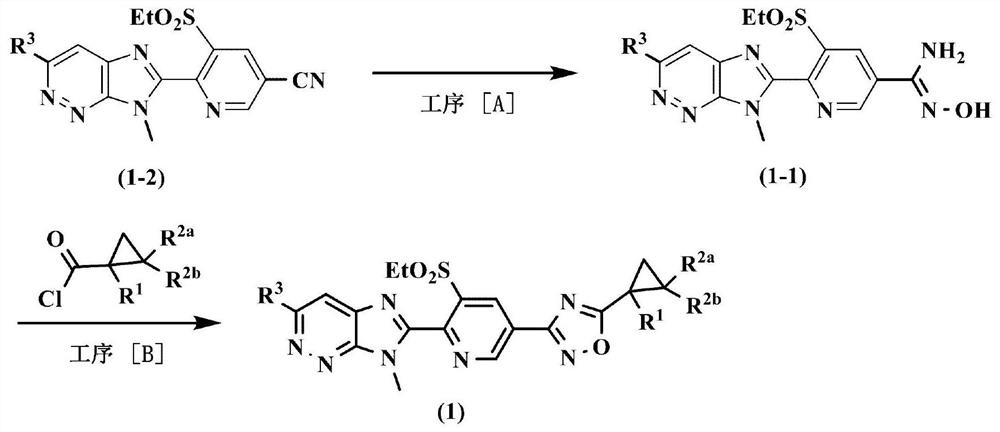
[0044] Production method of process [A]
[0045] The amidoxime compound represented by the general formula (1-1) can be produced by reacting a nitrile compound represented by the general formula (1-2) with hydroxylamines in the presence of a base and an inert solvent.
[0046] As the base used in this reaction, for example, inorganic bases such as sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, sodium bicarbonate, and potassium bicarbonate; acetates such as sodium acetate and potassium acetate; Potassium butoxide, sodium methoxide, sodium ethoxide and other alkali metal alkoxides; triethylamine, diisopropylethylamine, 1,8-diazabicyclo[5.4.0]undec-7-ene and other tertiary Amines; nitrogen-containing aromatic compounds such as pyridine and dimethylaminopyridine, etc., are usually used in the range of 1 to 10 times the mole of the compound represented by the general formula (1-2). .
[0047] Examples of hydroxylamines used in this reaction include ...
preparation Embodiment 1
[0178] Preparation Example 1.3-(5-(ethylsulfonyl)-6-(3-methyl-6-pentafluoroethyl-3H-imidazo[4,5-c]pyridazin-2-yl)pyridine- Preparation method of 3-yl)-5-(1-trifluoromethylcyclopropyl)-1,2,4-oxadiazole (compound number 1-2)
[0179] [chemical formula 3]
[0180]
[0181] N'-hydroxy-6-(3-methyl-6-pentafluoroethyl-3H-imidazo[4,5-c]pyridazine- 2-yl)-5-(ethylsulfonyl)pyridine-3-carboxamidine (0.15 g) was dissolved in THF (tetrahydrofuran, 3 mL), triethylamine (0.10 g), 1-trifluoromethylcyclopropane were added Formyl chloride (0.067g), stirred at room temperature for 1 hour. The reaction solution was concentrated under reduced pressure. This was dissolved in acetic acid (1 mL), and the acetic acid solution was dissolved in toluene (2 mL), and stirred at 120° C. for 1 hour. Saturated aqueous sodium carbonate solution was added, followed by extraction with ethyl acetate. The organic layer was dried using anhydrous magnesium sulfate, and concentrated under reduced pressure. Th...
preparation example 1
[0185]
[0186] Mix and dissolve the above substances evenly to make an emulsion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com