Main chain fluorocarbon alkali-resistant bipolar membrane and preparation method thereof
A bipolar membrane, fluorocarbon technology, applied in chemical instruments and methods, synthetic resin layered products, coatings, etc., can solve the problems of reducing the service life of bipolar membranes, easy to peel, etc., to achieve extended service life, strong resistance Alkaline, the effect of increasing the concentration of strong alkali resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
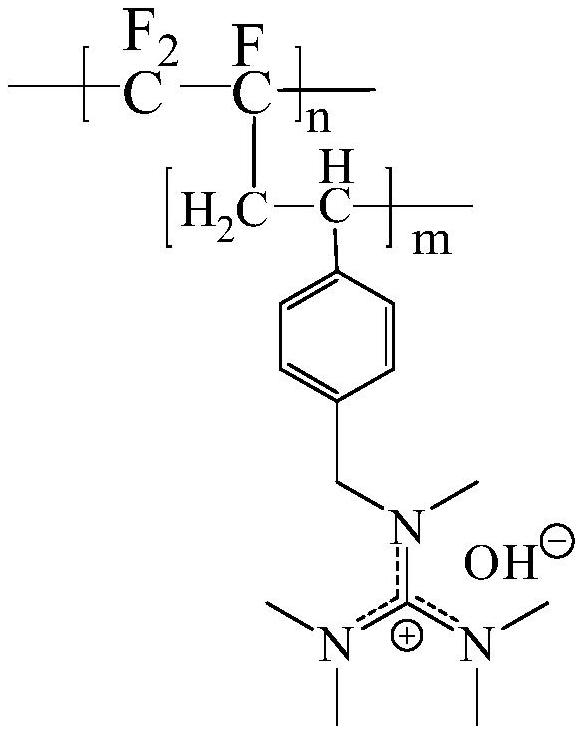
[0035] A main-chain fluorocarbon alkali-resistant bipolar membrane, including a cation-exchange membrane layer and an anion-exchange membrane layer. Both the cation-exchange membrane layer and the anion-exchange membrane layer use perfluorocarbon as the main chain; the chemical structural formula of the anion-exchange membrane layer is:
[0036]
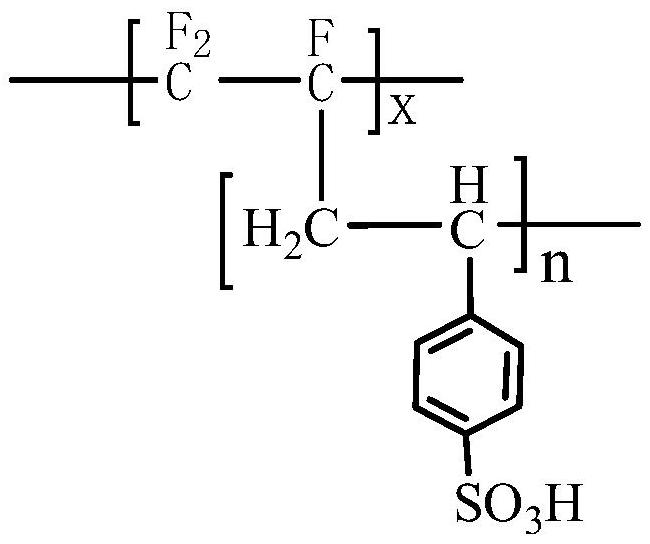
[0037] The chemical structural formula of the cation exchange membrane layer is:
[0038]
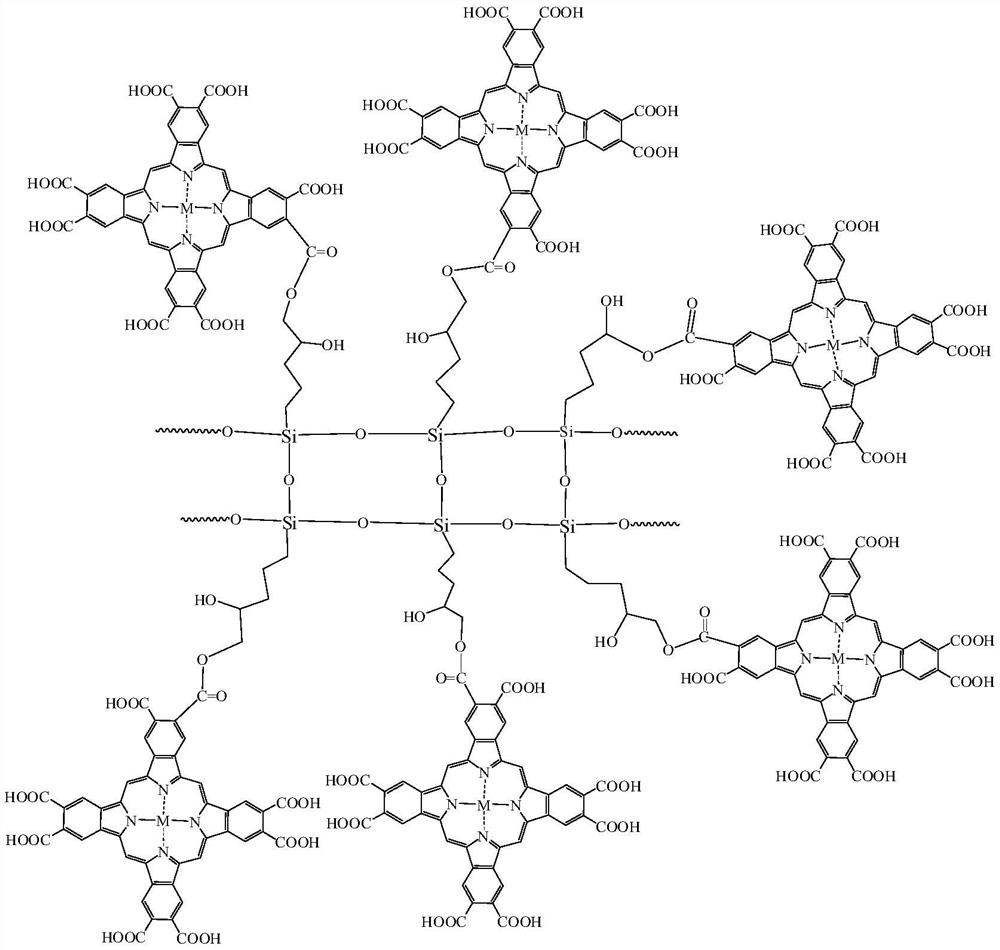
[0039] The chemical structural formula of the intermediate interface layer catalyst is:
[0040]
[0041] Wherein, x is the degree of polymerization of the polychlorotrifluoroethylene main chain, n is the degree of polymerization of the side chain containing the sulfonic acid group substituent, m is the degree of polymerization of the side chain containing the imidazole substituent, and n and m are not zero integer.
Embodiment 2
[0043] A method for preparing a main-chain fluorocarbon alkali-resistant bipolar membrane, comprising the following steps:
[0044]S1, in the present embodiment, polychlorotrifluoroethylene is poly(vinylidene fluoride-trifluorochloroethylene), and preparing poly(vinylidene fluoride-trifluorochloroethylene) grafted polymethylstyrene copolymer is to prepare poly(vinylidene fluoride-trifluorochloroethylene) Vinyl chloride) grafted polymethylstyrene copolymer, its preparation method is as follows: take 2.0g poly(vinylidene fluoride-chlorotrifluorovinyl chloride) and add reaction container, after dissolving with 60mL N-methylpyrrolidone, nitrogen gas deoxygenation According to the molar ratio of 1:40:1:2, poly(vinylidene fluoride-chlorotrifluoroethylene), methylstyrene, CuBr and pentamethyldiethylenetriamine (PMDETA) were added sequentially, and then nitrogen was introduced Exhaust the air in the reaction vessel, repeat the vacuum / nitrogen step 3 times, CuBr and pentamethyldiethyle...
Embodiment 3
[0059] A method for preparing a main-chain fluorocarbon alkali-resistant bipolar membrane, comprising the following steps:
[0060] S1, poly(vinylidene fluoride-trifluorochloroethylene) grafted polymethylstyrene copolymer was obtained as described in Example 1; 10 g of prepared poly(vinylidene fluoride-trifluorochloroethylene) grafted Methylstyrene copolymer P(VDF-co-CTFE)-g-PMS is dissolved in 150mL chlorobenzene, then add 12.0g brominating agent NBS and 1.2g initiator AIBN, make it boil under the condition of heating and stirring Reflux for 4 hours, continue to react for 2 hours after the color change occurs, and then stop heating; precipitate in anhydrous methanol to obtain flocs, remove impurities and dry to obtain the final bromomethylated polymer P(VDF-co-CTFE)-g- BrPMSt;
[0061] S2. Dissolve 0.50 g of the bromomethylated polymer P(VDF-co-CTFE)-g-BrPMSt obtained in step S1 in 10 mL of N,N-dimethylacetamide or tetrahydrofuran, and slowly add 1.5 times of The molar equi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com