Conjugated polymer containing quinoxaline structure as well as synthesis method and application of conjugated polymer
A technology of conjugated polymer and quinoxaline, applied in the field of conjugated polymer and its synthesis, can solve the problems of narrow absorption spectrum and low molar absorption coefficient, and achieve the effect of good response, optimized morphology and improved performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Prepare polymer D1, its synthetic route is as follows:
[0061]
[0062] Specific steps are as follows:
[0063] (1) Synthesis of compound 2: Cool the solution of n-butyl lithium (26.7mL, 64.0mmol, 1.0equiv) in anhydrous THF (100mL) to -78°C, heat it for 10min, and add Add 3-bromothiophene (compound 1, 6.0mL, 64.0mmol, 1.0equiv) dropwise, stir the mixture at -78°C for 4 hours, and record it as mixture solution a; Lithium bromide (5.56g, 64.0mmol, 1.0equiv) Slowly add cuprous bromide (9.19g, 64.0mmol, 1.0equiv) into anhydrous THF (150mL) solution, stir for 25 minutes until the solid dissolves, then cool the mixture to -40°C, and record it as mixture solution b; The mixture solution a was transferred to the mixture solution b, and then the system temperature was maintained at -78°C, which was recorded as mixture solution c; the THF (50mL) solution of oxalyl chloride (2.4mL, 28.8mmol, 0.45equiv) was cooled to -40 ℃, then it was transferred to the mixture solution c; t...
Embodiment 2
[0074] Prepare polymer D2, its synthetic route is as follows:
[0075]
[0076] Specific steps are as follows:
[0077] (1) Compound 2 was prepared according to the method of Example 1.
[0078] (2) Compound 3 was prepared according to the method of Example 1.
[0079] (3) Compound 4 was prepared according to the method of Example 1.
[0080] (4) Synthesis of Compound 6: Compound 4 (1.0g, 2.65mmol, 1.0equiv) was added to a dry round bottom flask, and then a solution of Compound 5 (576mg, 3.25mmol, 1.23equiv) in acetic acid (180mL) was added into the reaction bottle, heated the mixture solution to 50°C, stirred and reacted at 400rpm for 19 hours, and the reaction was carried out under nitrogen atmosphere; after the reaction, the mixture solution was naturally cooled to room temperature, and the solid was collected by filtration and washed with methanol, and then dried The crude product was obtained as a tan solid, that is, compound 6, which was directly used in the next s...
Embodiment 3
[0088] Prepare polymer D3, its synthetic route is as follows:
[0089]
[0090] Specific steps are as follows:
[0091] (1) Compound 2 was prepared according to the method of Example 1.
[0092] (2) Compound 3 was prepared according to the method of Example 1.
[0093] (3) Compound 4 was prepared according to the method of Example 1.
[0094] (4) Synthesis of Compound 6: Compound 4 (1.0g, 2.65mmol, 1.0equiv) was added to a dry round bottom flask, and then a solution of Compound 5 (352mg, 3.25mmol, 1.23equiv) in acetic acid (180mL) was added into the reaction bottle, heated the mixture solution to 50°C, stirred and reacted at 400rpm for 19 hours, and the reaction was carried out under nitrogen atmosphere; after the reaction, the mixture solution was naturally cooled to room temperature, and the solid was collected by filtration and washed with methanol, and then dried The crude product was obtained as a tan solid, that is, compound 6, which was directly used in the next s...
PUM
| Property | Measurement | Unit |
|---|---|---|
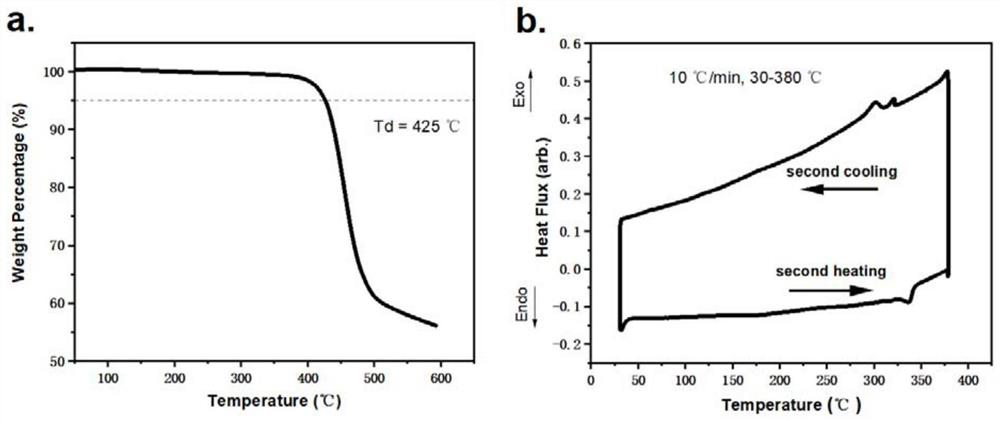
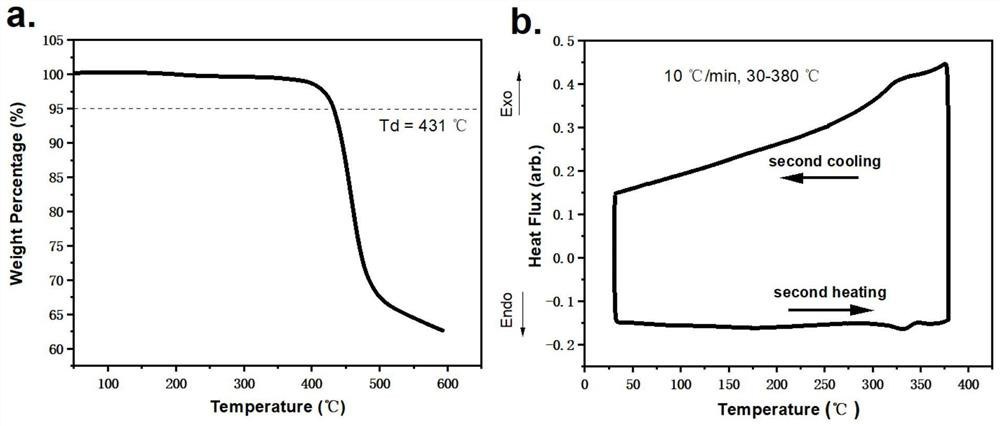
| thermal decomposition temperature | aaaaa | aaaaa |
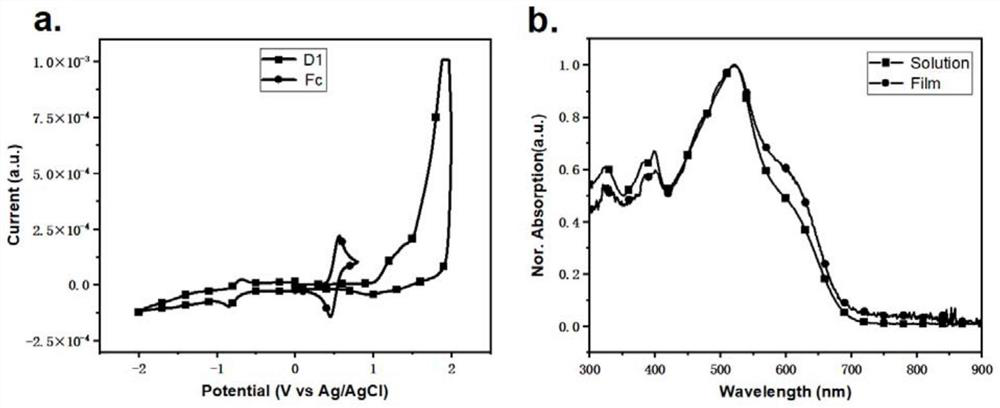
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com