Water-soluble polyester monomer as well as preparation method and application thereof
A water-soluble polyester and monomer technology, applied in the field of water-soluble polyester, can solve the problems of WPET environmental protection and non-toxic and side effects reduction, poor water resistance of coating film, storage stability, poor water solubility, etc., and achieve good adaptability , Improve water resistance, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Epoxycyclohexane, oxalic acid and ethylene glycol titanium are mixed in the refining kettle in a molar ratio of 1:0.2:0.01 and stirred, N 2 Protected, reacted at 30 °C for 2 h, washed, concentrated and purified the epoxycyclohexane functional monomer (B1), the conversion rate was 99.4%, and the NMR H spectrum was as follows figure 1 shown. 1 H NMR (500 MHz, Chloroform ) δ 4.24 (s, 13H), 3.65 (s, 16H), 3.51–3.35 (m, 2H), 3.51–3.06 (m, 49H), 1.94 (d, J = 5.0 Hz, 34H), 1.80 (s, 12H), 1.75 (s, 14H), 1.68 (s, 22H), 1.61 (d, J = 10.0 Hz, 31H), 1.31 (s, 8H), 1.30 – 1.16 (m, 131H), 1.10 (s, 11H).
[0031] The structural formula of epoxy cyclohexane functional monomer (B1) is:
[0032] .
Embodiment 2
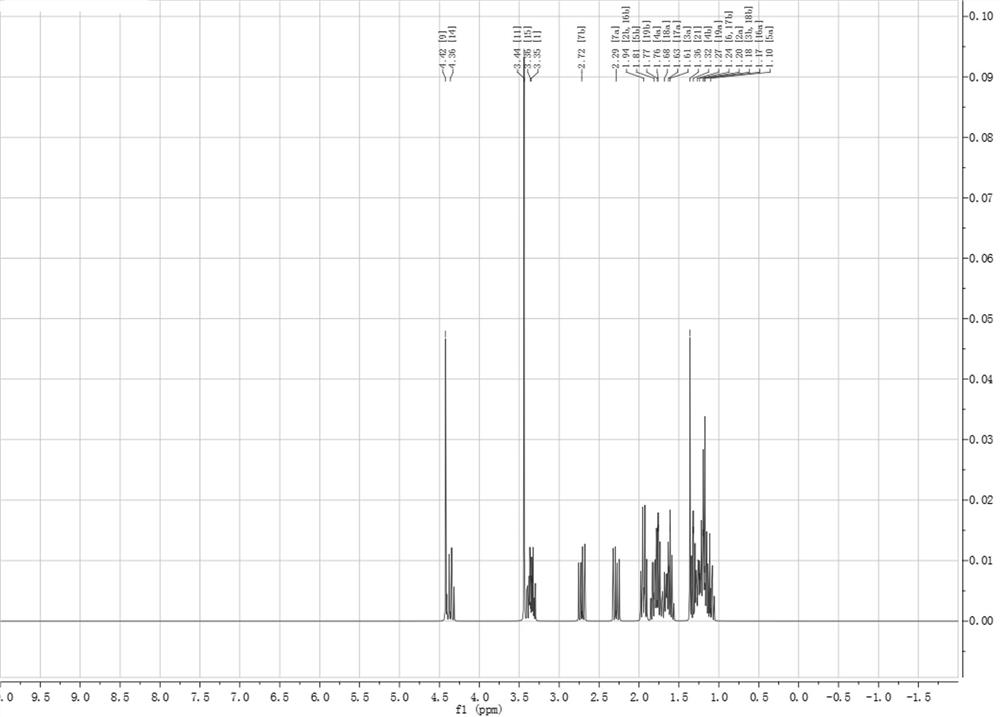
[0034] Epoxycyclohexane, malonic acid and ethylene glycol antimony were mixed in the refining kettle in a molar ratio of 1:0.5:0.1 and stirred, N 2 Protected, reacted at 50 °C for 4 h, washed, concentrated and purified epoxycyclohexane functional monomer (B2), the conversion rate was 99.6%, and the NMR H spectrum was as follows figure 2 shown. 1 H NMR (500 MHz, Chloroform ) δ 4.42 (s, 7H), 4.36 (s, 6H), 3.44 (s, 13H), 3.35 (d, J = 6.7 Hz, 9H), 2.72 (s, 5H), 2.29 (s, 7H), 1.94 (s, 9H), 1.81 (s, 6H), 1.76 (d, J = 5.0 Hz, 13H), 1.68 (s, 5H), 1.62 (d, J = 10.0 Hz, 12H), 1.36 (s, 8H), 1.32 (s, 4H), 1.25 (d, J = 15.0 Hz, 17H), 1.22 – 1.15 (m, 27H), 1.10 (s, 8H).
[0035] The structural formula of epoxy cyclohexane functional monomer (B2) is:
[0036] .
Embodiment 3
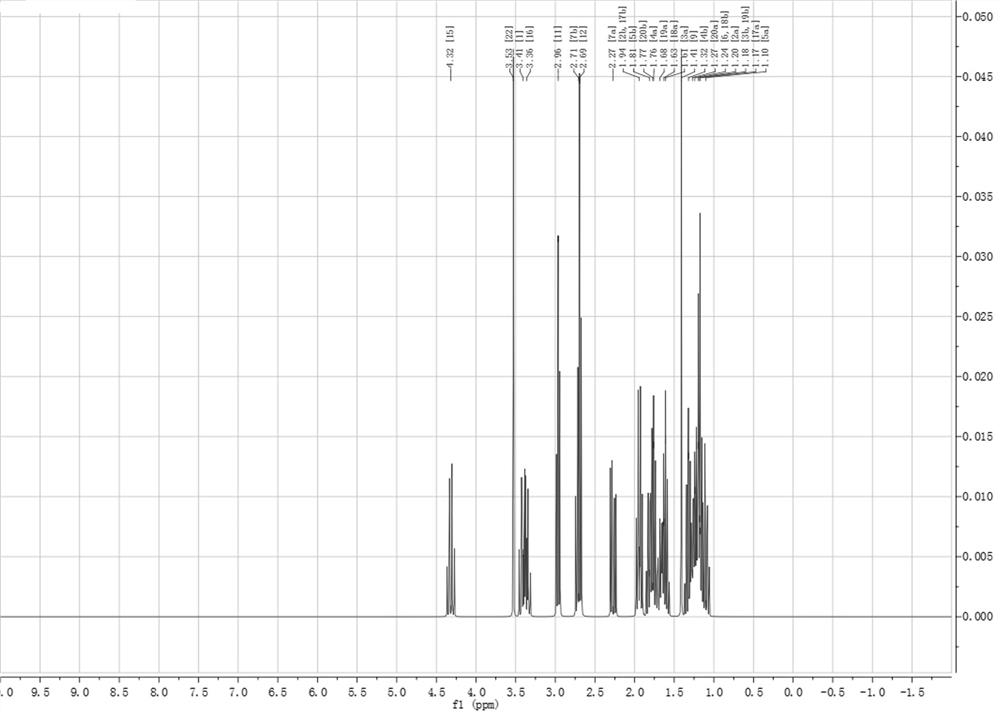
[0038] Epoxycyclohexane, succinic acid and imidazole column aromatic hydrocarbon ionic liquid were mixed in the refining kettle in a molar ratio of 1:0.3:0.08 and stirred, N 2 Protected, reacted at 40 °C for 3 h, washed, concentrated and purified the epoxycyclohexane functional monomer (B3), the conversion rate was 99.3%, and the NMR H spectrum was as follows image 3 shown. 1 H NMR (500 MHz, Chloroform ) δ 4.32 (s, 10H), 3.53 (s, 10H), 3.41 (s, 12H), 3.36 (s, 5H), 2.96 (s, 13H), 2.70 (d, J = 6.3 Hz, 22H), 2.27 (s, 10H), 1.94 (s, 14H), 1.81 (s, 9H), 1.76 (d, J = 5.0 Hz, 20H), 1.68 (s, 8H), 1.62 (d, J = 10.0 Hz, 18H), 1.41 (s, 10H), 1.32 (s, 6H), 1.25(d, J = 15.0 Hz, 25H), 1.22 – 1.15 (m, 41H), 1.10 (s, 7H).
[0039] The structural formula of epoxy cyclohexane functional monomer (B3) is:
[0040] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com