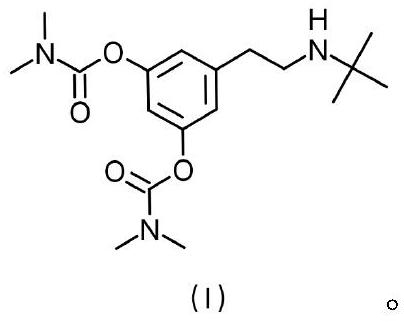
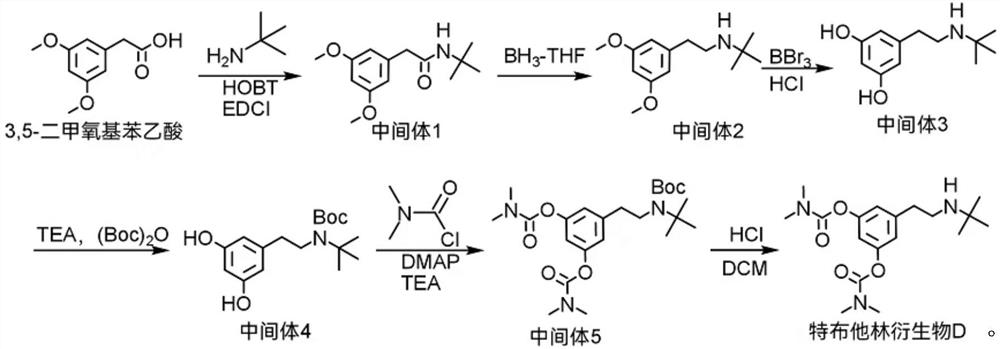
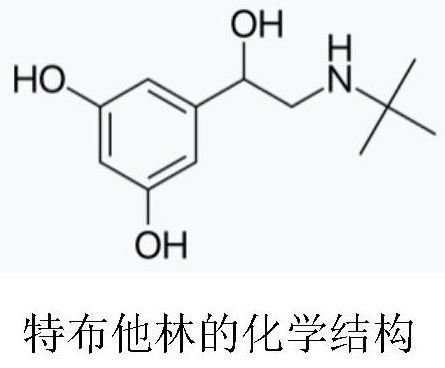
Terbutaline derivative D as well as preparation method and application thereof
A technology for terbutaline derivatives, applied in the field of terbutaline derivative D and its preparation, capable of solving the problems of not providing the use of terbutaline derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take a 250ml three-necked flask, add 9.8g of 3,5-dimethoxyphenylacetic acid, 7.3g of tert-butylamine, 50g of dichloromethane, 6.7g of HOBT, and 11.5g of EDCI, react at room temperature, and monitor by HPLC until the reaction is complete. The reaction was added dropwise to 100 g of water, extracted with dichloromethane, the organic layer was washed with water until neutral, allowed to stand for separation, and the organic phase was concentrated under reduced pressure to obtain about 13 g of Intermediate 1 with a liquid phase purity of 98.0%.
Embodiment 2
[0036] Take a 250ml three-necked flask, dissolve the above-mentioned intermediate 1 with 13g of tetrahydrofuran, dropwise add a solution of borane in tetrahydrofuran (150ml, 1.0M), stir for 15 minutes after the dropwise addition, and then reflux for reaction until the reaction is complete. Then 20 g of methanol was added to continue refluxing for 8 h, and the solvent was removed under reduced pressure. 50 g of ethyl acetate and 100 g of water were added to the residue, then 2 ml of concentrated hydrochloric acid was added to adjust the pH to 3, the mixture was left to stand for separation, and the organic layer was discarded. Add 50 g of ethyl acetate to the aqueous layer, adjust the pH to 7.5-8.5 with sodium hydroxide, stand for separation, discard the aqueous layer, and wash the organic layer with water until neutral. The organic phase was concentrated under reduced pressure to obtain Intermediate 2 with a purity of 95.0%.
Embodiment 3
[0038] Take a 250ml three-necked flask, add Intermediate 2 and 60g of dichloromethane, cool to below 0°C, add 25g of boron tribromide dropwise, stir for 15 minutes after the dropwise addition, and then stir for 3h below 0°C until the reaction is complete. The reaction solution was poured into 100 g of ice water, left to stand for separation, and the organic layer was discarded. Add 50 g of ethyl acetate to the aqueous layer, adjust the pH to 7.5-8.5 with sodium hydroxide, stand for separation, discard the aqueous layer, and wash the organic layer with water until neutral. The organic phase was concentrated under reduced pressure to obtain intermediate 3 with a purity of 97.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com