Endotoxin latex microspheres as well as preparation method and application thereof
An endotoxin and microsphere technology, applied in the preparation of microspheres, microcapsule preparations, instruments, etc., can solve the problems of large deviation of experimental results, large interference, and inability to use, achieve good stability, enhance scattering signals, and improve detection. The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
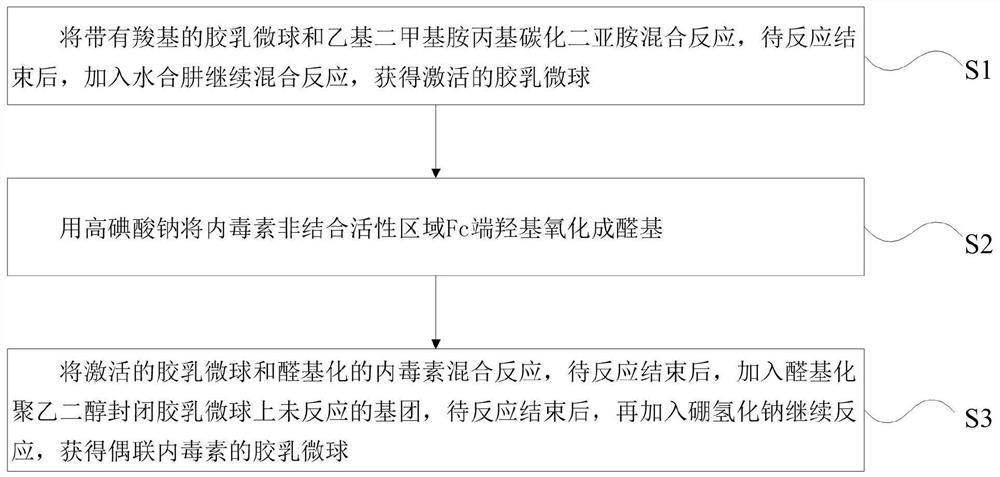
[0031] combine figure 1 As shown, the first aspect of the embodiment of the present application provides a method for preparing endotoxin latex microspheres, comprising the following steps:
[0032] Step S1, mixing and reacting latex microspheres with carboxyl groups and ethyldimethylaminopropyl carbodiimide, after the reaction is completed, adding hydrazine hydrate to continue the mixing reaction to obtain activated latex microspheres;
[0033] Step S2, using sodium periodate to oxidize the hydroxyl group at the Fc end of the non-binding active region of the endotoxin into an aldehyde group;
[0034] Step S3, mixing the activated latex microspheres with the aldehydelated endotoxin, and adding aldehydelated polyethylene glycol to seal the unreacted groups on the latex microspheres after the reaction, and then adding Sodium borohydride continues to react to obtain latex microspheres coupled with endotoxin.
[0035] In the examples of the present application, the Fc end of the...
Embodiment 1
[0061] This embodiment provides a method for preparing endotoxin latex microspheres, comprising the following steps:
[0062] (1) Take 0.1 mg of carboxylated latex microspheres with a particle size of 130-200 nm (commercialized carboxylated latex microspheres from Bangs Lab Company), wash twice with 10-50 mmol / LMES buffer (pH 4.0); add Add a certain volume of buffer solution, add 0.1mg of EDC (purchased by sigma company), shake and react at room temperature for 15-30min, after the reaction, centrifuge to remove the supernatant, wash with buffer solution twice, add excess hydrazine hydrate (N 2 h 4 ) solution, so that the final concentration of the carboxylated latex microspheres in the mixed solution is about 0.1% w / v, shake and react at normal temperature for 90-120min, after the reaction finishes, centrifuge to remove the supernatant, and use 10-50mmol / L boron Salt buffer (pH is 8.0) was washed twice to obtain activated latex microspheres;
[0063] (2) Take 0.5uL 5mg / mL en...
Embodiment 2
[0066] This example provides a method for verifying the non-specific recognition of endotoxin latex microspheres, prepared respectively according to the preparation method in Example 1:
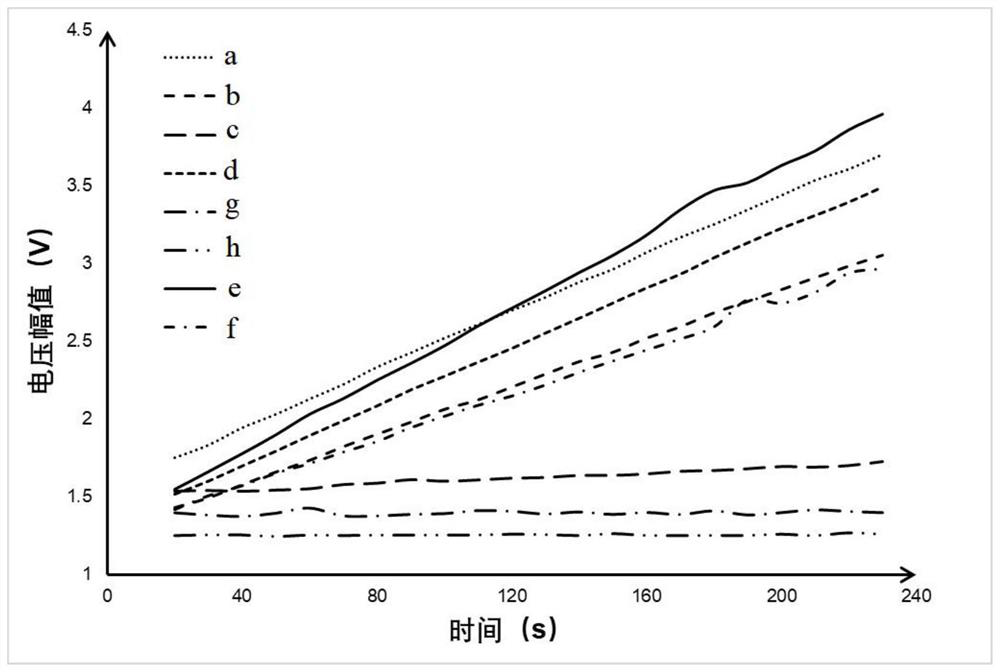
[0067] (a) Bare spheres: carboxylated latex microspheres;
[0068] (b) EDC-activated latex microspheres: refer to the latex microspheres only activated with EDC in the embodiment 1 step (1);
[0069] (c) grafted hydrazine hydrate latex microspheres after activation: refer to the activated latex microspheres prepared in Example 1 step (1);
[0070] (d) Latex microspheres grafted with hydrazine hydrate and endotoxin after activation: refers to the reaction product after the mixed reaction between the aldylated endotoxin and the activated latex microspheres at room temperature in step (3) of Example 1;
[0071] (e) Latex microspheres grafted with hydrazine hydrate and endotoxin after activation and blocked with PEG-550: refer to the coupled endotoxin latex microspheres blocked with aldehydelate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com