Carbazole derivative based on epoxybutane as well as preparation method and application of carbazole derivative
A technology of butylene oxide and carbazole, which is applied in the field of organic optoelectronics, can solve problems such as damage and interlayer miscibility, and achieve the effects of simple process, good solvent resistance and thermal stability, and improved hole transport capability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
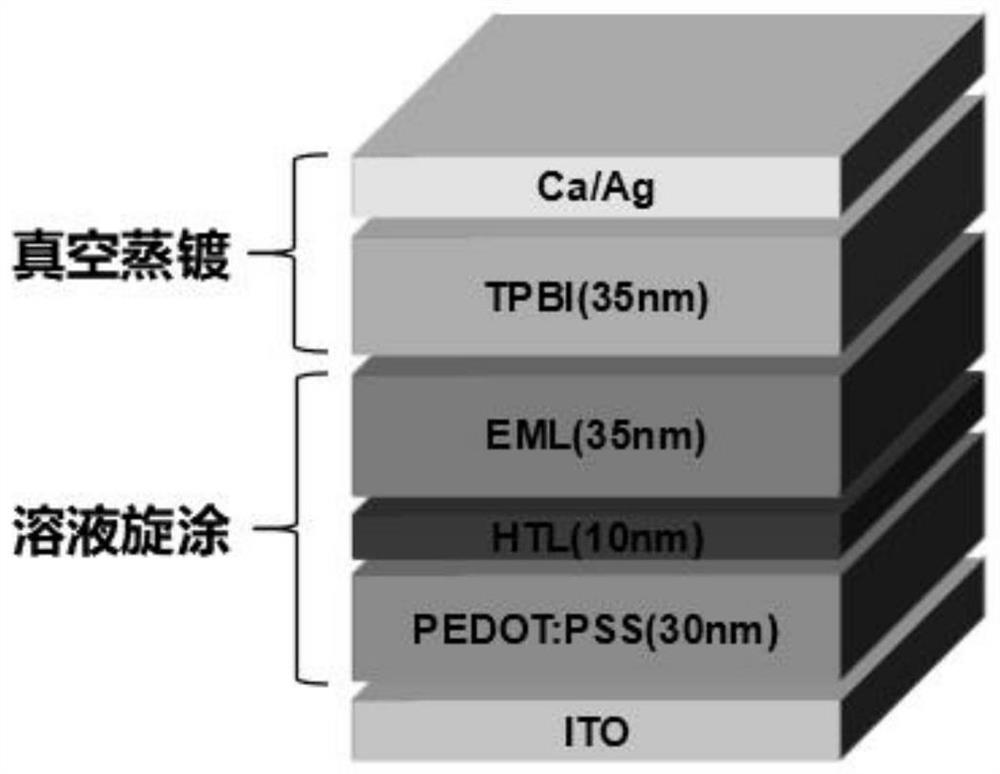
Image
Examples
Embodiment 1
[0042] Example 1: Oxe-DCzTPA (3,5-bis(4-((6-((3-ethyloxetan-3-yl)methoxy)hexyl)oxy)-9H-carbazole -9-yl)-N,N-diphenylaniline) preparation method
[0043] The synthetic route of described Oxe-DCzTPA is as follows:
[0044]
[0045] The Oxe-DCzTPA preparation process and NMR data are characterized by:
[0046] (1) Preparation of Compound 1: Dissolve 3g of compound 9H-carbazol-4-ol, 4.5g of potassium carbonate and 0.528g of compound TBABr in 160mL of ethyl acrylate for 1h at room temperature, then add 3.06mL of compound CH 3 I obtain compound 1, 3.01g, productive rate 94% after heating to reflux 24h: 1 H NMR (400MHz, Chloroform-d) δ8.32 (d, J = 7.8Hz, 1H), 8.03 (s, 1H), 7.43–7.30 (m, 3H), 7.26–7.21 (m, 1H), 7.08 – 7.00(m,1H),6.68(d,J=8.0Hz,1H),4.08(s,3H).
[0047] (2) Preparation of compound 2: Add 2 g of compound 1, an appropriate amount of 2.24 g of compound t-BuOK and 576 μL of 1-bromo-3,5-difluorobenzene into 10 mL of DMSO to dissolve in sequence, and heat to reflux for...
Embodiment 2
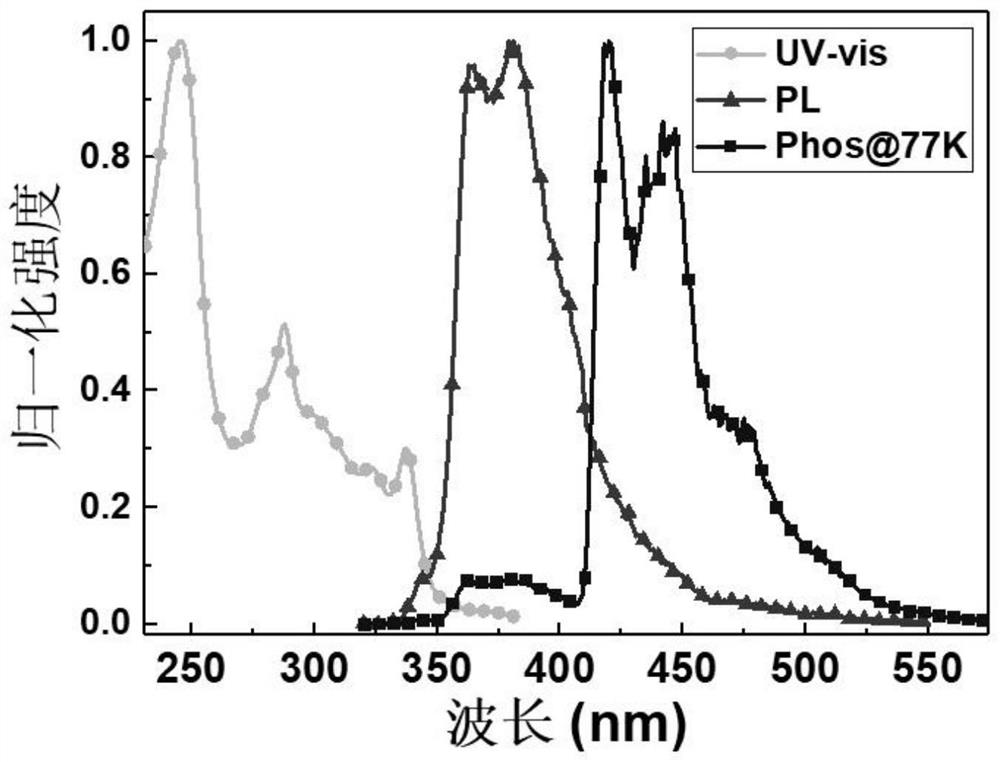
[0054] Embodiment 2: the photophysical property of Oxe-DCzTPA film
[0055] figure 2 The ultraviolet absorption spectrum, fluorescence spectrum and low temperature phosphorescence spectrum of the compound Oxe-DCzTPA in the film are given. The compound was dissolved in dichloromethane at a mass concentration of 4 mg / mL to form a film, and then its spectrum, fluorescence spectrum and low-temperature phosphorescence spectrum were measured. In the film, the ultraviolet absorption peaks of the compound are located at 246, 288 and 337nm respectively, the fluorescence spectrum of the compound has a peak at 379nm, and a slightly weaker peak at 363nm, and it can be known from the low-temperature phosphorescence spectrum that it is short at 420nm According to the calculation of the starting point on the wavelength side, the compound has a high triplet energy of 2.95eV, and the high triplet energy also makes it have good exciton blocking ability, which can be used as an exciton blockin...
Embodiment 3
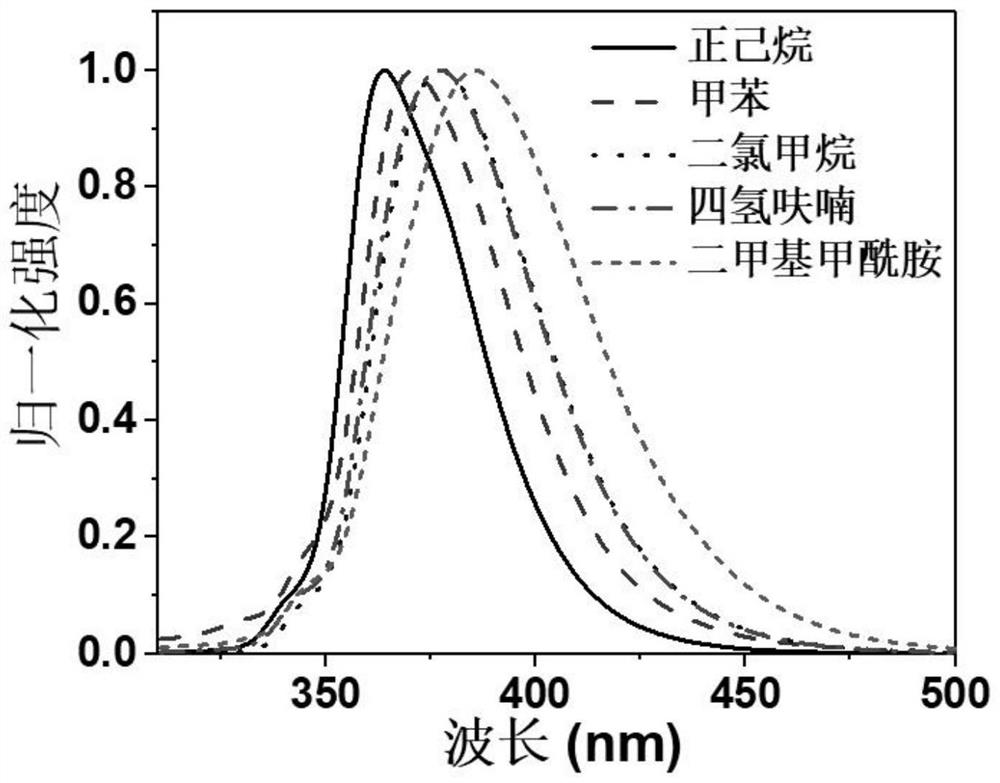
[0056] Embodiment 3: the photophysical property of Oxe-DCzTPA solution
[0057] image 3 The fluorescence spectra of the compound Oxe-DCzTPA in different solutions are given, and the compounds are formulated into 10 -5 mol / L n-ethane, toluene, dichloromethane, tetrahydrofuran, dimethylformamide solvent dilute solution, pipette a certain volume of the compound solution into a fluorescence cuvette, and test its fluorescence spectrum. The fluorescence emission positions of the compounds in n-ethane, toluene, dichloromethane, tetrahydrofuran, and dimethylformamide solutions are all the same, and the emission wavelengths of the spectral peaks are 363nm, 371nm, 376nm, 376nm, and 384nm in sequence.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com