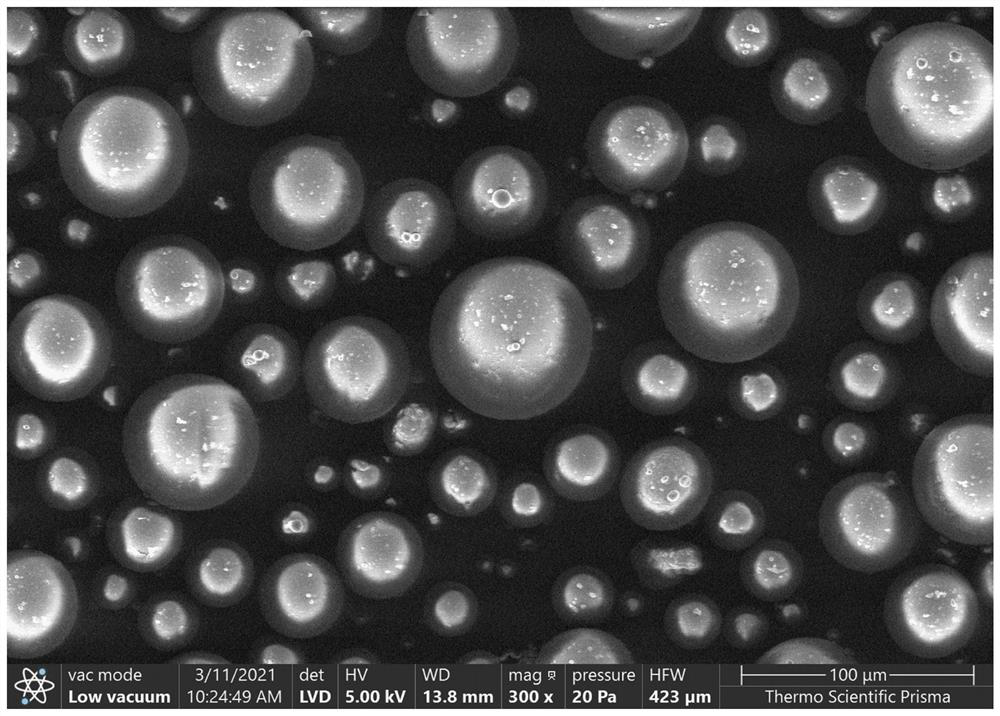
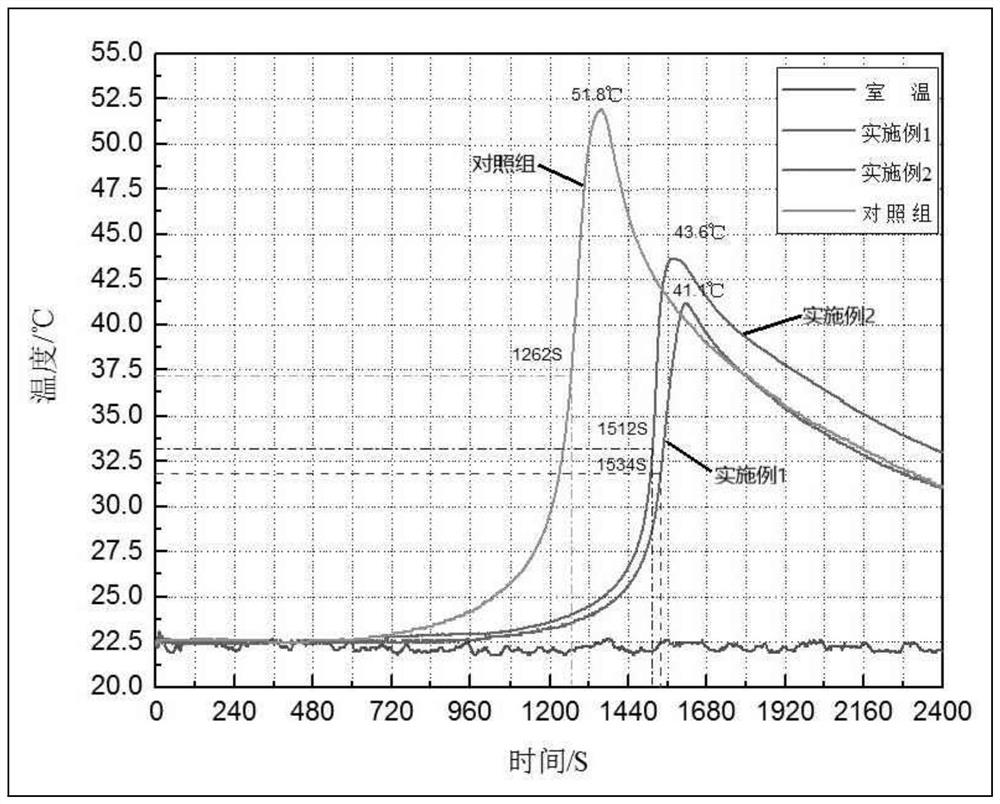
Methyl methacrylate copolymer microsphere encapsulated with initiator, preparation method and injectable bone cement
A technology of methyl methacrylate and copolymers, which can be used in drug delivery, pharmaceutical formulations, prostheses, etc., and can solve problems such as unfavorable clinical operations for doctors, necrosis, and short curing time of PMMA bone cement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] One, the preparation of the methyl methacrylate copolymer microsphere of encapsulation initiator:
[0049] S1, preparation of methyl methacrylate copolymer suspension containing initiator:
[0050] Under the condition of rotating speed of 400r / min, 2 parts of hydroxyethyl cellulose was added into 500 parts of deionized water and stirred to dissolve. After the hydroxyethyl cellulose is completely dissolved, add 5 parts of dibenzoyl peroxide (BPO) with a mass fraction of 75% and 300 parts of methyl methacrylate monomer, raise the temperature to 80°C and keep it warm for 60 minutes, then raise the temperature To 92 ℃ ~ 95 ℃ and continue to keep warm for 30 minutes. The resulting suspension was cooled to room temperature to obtain a methyl methacrylate copolymer suspension slurry. After analysis, the solid content in the obtained methyl methacrylate copolymer suspension slurry was 37.5%, and the residual amount of BPO was 0.86%.
[0051] S2, preparation of methyl methacr...
Embodiment 2
[0066] One, the preparation of the methyl methacrylate copolymer microsphere of encapsulation initiator:
[0067] S1, preparation of methyl methacrylate copolymer suspension containing initiator:
[0068] With the method of S1 among the embodiment 1.
[0069] S2, preparation of methyl methacrylate homopolymer emulsion:
[0070] S2.1 Preparation of monomer emulsion:
[0071] Add methyl methacrylate, deionized water, and sodium lauryl sulfate into a round-bottomed flask at a ratio of 100:30:0.5, and keep stirring evenly to prepare a mixed monomer emulsion.
[0072] S2.2 Preparation of methyl methacrylate homopolymer emulsion:
[0073] Add 125 parts of deionized water into a four-neck flask equipped with a reflux tube, a thermometer, an addition funnel and a stirrer, heat it in a water bath to 80°C, and fill it with nitrogen for protection at a speed of 150r / min. Add 50 parts of the monomer emulsion prepared by S2.1 into a four-necked bottle, then add 10 parts of 2wt% potassi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com