Preparation method of nitrogen trifluoride and nitrogen trifluoride mixed gas
A nitrogen trifluoride and mixed gas technology, which is applied in the field of chemical engineering, can solve the problems of insufficient preparation efficiency, many by-products, and unfavorable use of fluorine elements, so as to overcome the low fluorine content, increase the content of F elements, and react The effect of high conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
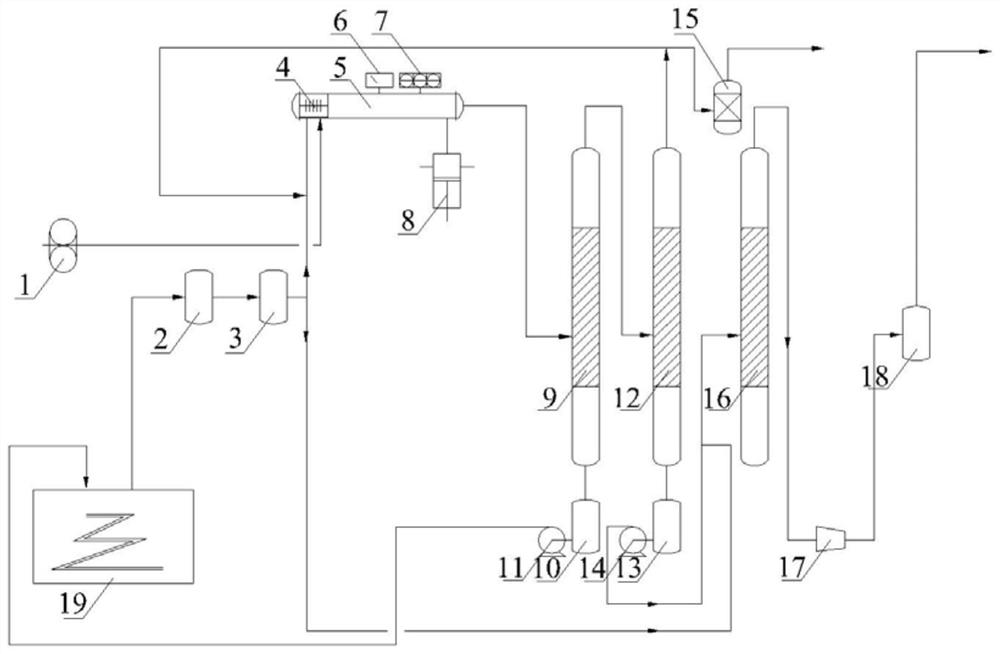
[0034] The fluorination reactor used in this embodiment is a circular pipeline fluorination reactor, and the reaction tube is 4.8m long and has an inner diameter of 65mm;
[0035] Before the reaction, the pressure in the reaction tube was pumped to 180Pa by a vacuum pump, the temperature was normal temperature, and then the reaction gas was slowly added to react, and the molar ratio of ammonia to fluorine was controlled to be 1:7.2, and the mass content of ammonia in the fluorine-containing mixed gas is 5.0%; in the specific reaction process, the ammonia gas enters the above-mentioned fluorination reactor after adjusting the flow rate to 2.0g / h, and the fluorine-containing mixed gas (the mass percentage of fluorine gas is 85%, and the rest is nitrogen) adjusts the flow rate to 38.0 g / h, enter the fluorination reactor for reaction;
[0036] The mixed gas prepared by the fluorination reactor is pressurized to a pressure of 0.15 MPa, followed by the first-stage condensation separ...
Embodiment 2
[0040] The difference between this example and Example 1 is that the molar ratio of ammonia gas to fluorine gas is 1:6.6, and the mass content of ammonia gas in the fluorine-containing mixed gas is 5.0%; during the reaction, the flow rate of ammonia gas is adjusted to 2.0 g / g Enter the above-mentioned fluorination reactor after h, and the fluorine-containing mixed gas (the mass percentage of fluorine gas is 78%, and the rest is nitrogen) adjusts the flow rate to 38.0g / h, and enters the fluorination reactor for reaction;
[0041] The mixed gas prepared by the reaction is pressurized to a pressure of 0.25 MPa, and the first stage and the second stage condensation separation are carried out in turn, wherein the first stage condensation temperature is controlled at -25 ° C, and the condensation temperature of the second stage separation device is Controlled at -145℃;
[0042]The liquid after secondary condensation is nitrogen trifluoride liquid; the nitrogen trifluoride sample is ...
Embodiment 3
[0045] The difference between this example and Example 2 is that the molar ratio of ammonia gas to fluorine gas is controlled to be 1:11.0, and the mass content of ammonia gas in the fluorine-containing mixed gas is 2.9%; in the specific reaction process, the flow rate of ammonia gas is adjusted to After 1.2g / h, enter the above-mentioned fluorination reactor, and the fluorine-containing mixed gas (the mass percentage of fluorine gas is 75%, and the rest are nitrogen), adjust the flow rate to 39.5g / h, and enter the fluorination reactor to react;
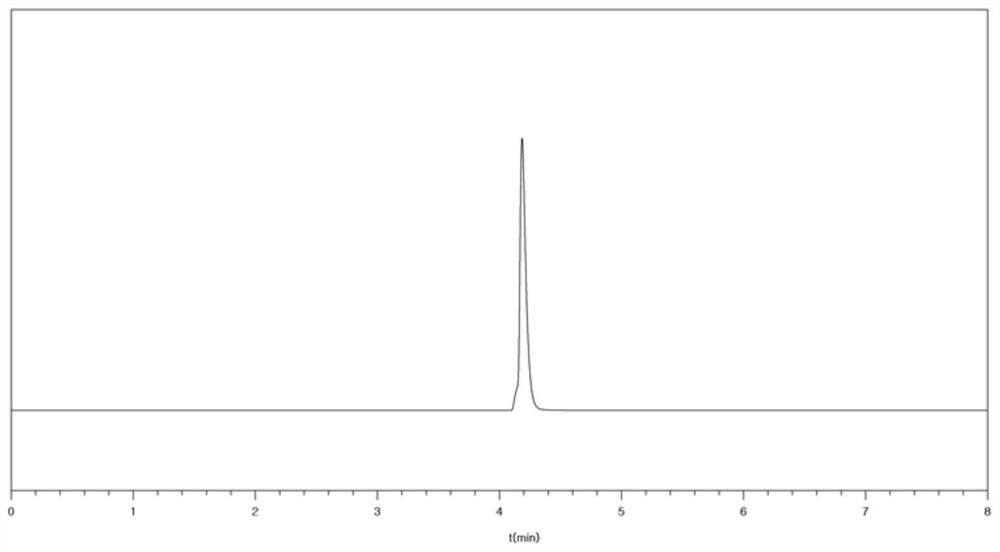
[0046] The purity of the obtained nitrogen trifluoride was 99.999% as detected by gas chromatography.
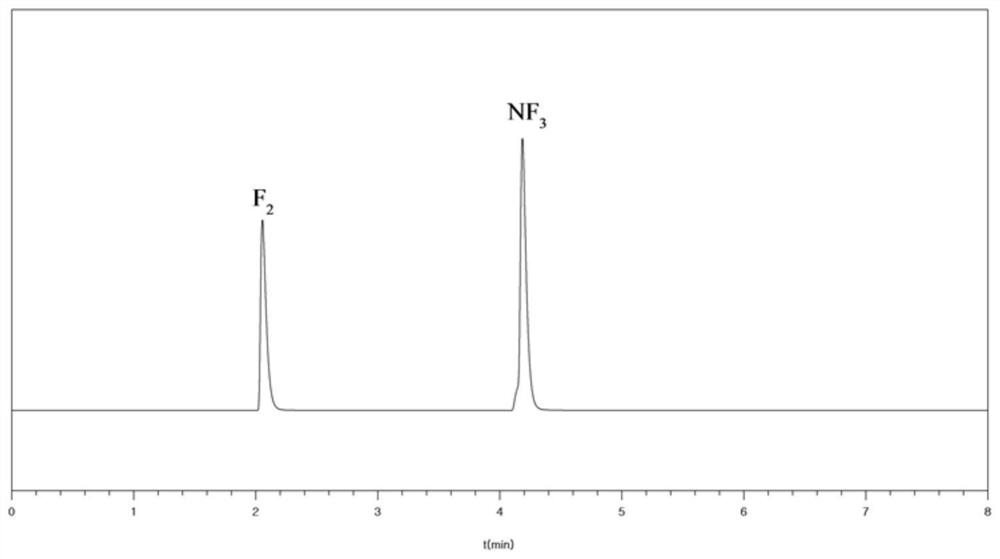
[0047] Mix and modulate fluorine gas and nitrogen trifluoride, and control the temperature of the mixed gas conditioning device to -20°C to ensure that nitrogen trifluoride is completely vaporized and mixed with fluorine gas. A mixed gas of nitrogen fluoride / 35% fluorine gas, the F / N atomic ratio of the mixed gas is 5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com