Alpha9alpha10nAChR inhibitory active peptide and application thereof
A technology of active polypeptides and active monomers, which can be used in the fields of peptides, peptide sources, allergic diseases, etc., and can solve the problems of low yield, difficult synthesis, and difficult purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1: Synthetic method of polypeptide
[0102] The polypeptide in the present invention can be synthesized by Fmoc or Boc solid-phase synthesis method.
[0103] 1.1 Synthesis of linear peptides
[0104] Taking Fmoc solid-phase synthesis as an example, the synthesis steps are as follows:
[0105] 1. Weigh 0.2 mmol RAM resin, place it in a solid-phase reaction tube, and add equal proportions of N,N-dimethylformamide (DMF) and dichloromethane (DCM) to swell overnight;
[0106] 2. Add 20% piperidine and react in a shaking table at 37 degrees for 0.5h to remove the Fmoc protecting group of the amino group on the resin. After the reaction is completed, wash the resin three times with DMF and DCM respectively;
[0107] 4. Add 5eq of the C-terminal first amino acid, 4.5eq of HCTU and 8eq of DIPEA and place it on a shaker at 37 degrees to carry out the condensation reaction of the first amino acid. After the 1h reaction is completed, wash the resin three times with DMF an...
Embodiment 2
[0129] Example 2: Electrophysiological Activity Test Method
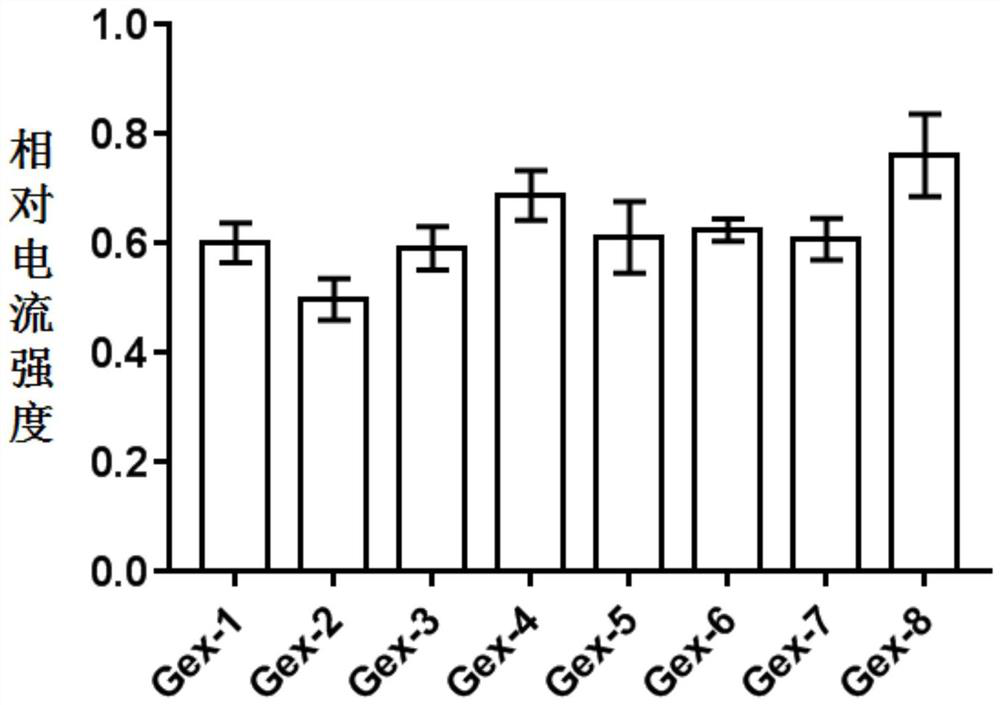
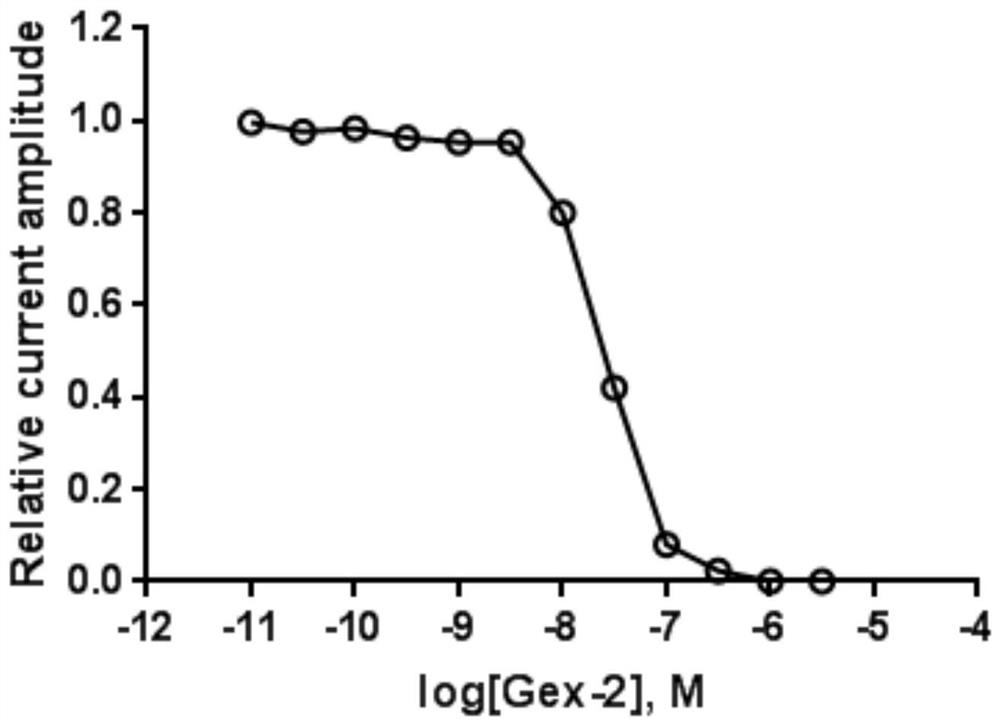
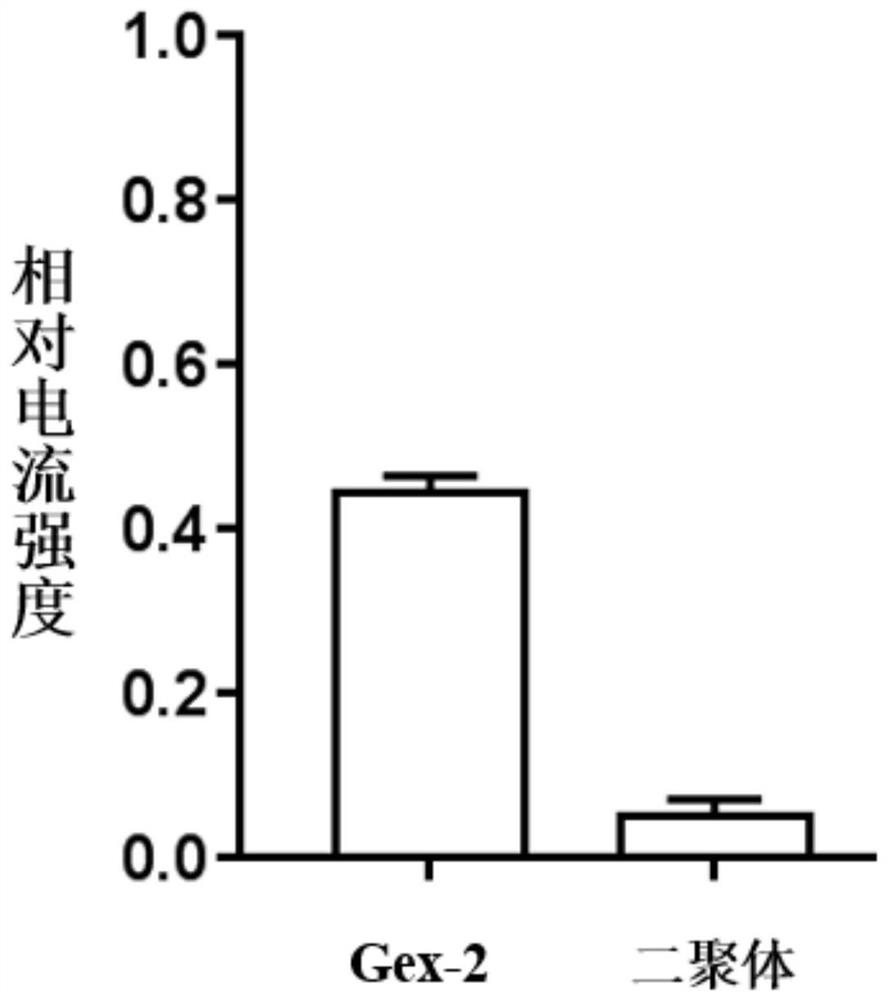
[0130] In order to verify the inhibitory activity of the active polypeptide on the human α9α10 acetylcholine receptor, the relative inhibitory intensity of the polypeptide on the acetylcholine-evoked peak current on the human α9α10 acetylcholine receptor was detected. Methods as below:
[0131] The cRNA of human α9α10 acetylcholine receptor was prepared by in vitro transcription kit, and its concentration was measured by OD value under UV260m. Xenopus oocytes were dissected and collected, and the cRNAs of the two subunits were injected into frog eggs on the first and second day, and the injection amount was 5ng, and cultured in ND-96. Oocytes expressing the α9α10 acetylcholine receptor were tested for electrophysiological activity 1-4 days after injection. The specific test method is as follows: 1 xenopus oocyte injected with cRNA was placed in a 30uL Sylgard recording tank with a diameter of 4mm and a depth of 2m...
Embodiment 3
[0132] Example 3: Construction of an animal model of in vivo analgesic activity
[0133] Animal models were used to evaluate the effect of active peptides on pain perception. The analgesic activity was tested using the rat sciatic nerve chronic compression injury (CCI) model.
[0134] The modeling process is as follows: after the rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium, the hair of the right leg was removed, the skin of the right lower limb was incised 1-2 cm under aseptic conditions, the muscles and fascia were bluntly separated, and the trunk of the sciatic nerve was exposed. , use 4-0 chrome catgut to tie four knots with a spacing of 1mm, and the tightness is required until the rat's toes twitch slightly, so as not to affect the blood supply of the epineurium, after the ligation is completed, use 40,000 units The wound was cleaned with sodium penicillin and finally sutured, and 40,000 units of sodium penicillin was intramuscularly inj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com