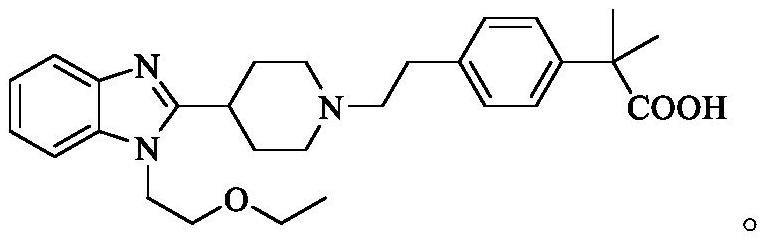
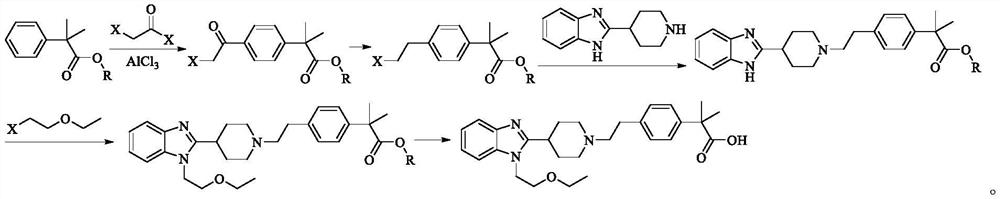
Bilastine intermediate compound and preparation method thereof
A bilastine and compound technology, applied in the field of drug synthesis, can solve problems such as complex operation, long reaction route, and difficult purification of products, and achieve the effects of simple synthesis process, high yield and purity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083]At room temperature, 1-(2-ethoxyethyl)-2-(piperidine-4-yl)-1H-benzo[d]imidazole (54.68g, 0.20mol), triethylamine (44.52g, 0.44mol) was added to N, N-dimethylformamide (300ml), added (E)-1,2-dibromoethylene (39.03g, 0.21mol) of N, N-dimethylformamide (100ml) solution, temperature control 80 ~ 85 ° C reaction, after the detection of the reaction, will The reaction liquid was cooled to room temperature, filtered, the filtrate was added to purified water (2000ml), dichloromethane (600ml×3) extracted, combined organic phase, saturated brine (500ml×2) washed, anhydrous sodium sulfate dried, filtered filtrate was concentrated to dry I-1, yield 85.3%, HPLC purity 99.63%.
[0084] Synthesis of compound I-2
Embodiment 2
[0086] Argon protects the room temperature, the compounds I-1 (37.83g, 0.10 mol), binborate pinaol ester (SM-1, 33.01 g, 0.13 mol), potassium acetate (15.70 g, 0.16 mol), Pd (PPh 3 ) 4 (5.78g, 5.0mmol) was added to dimethyl sulfoxide (250ml), the temperature control was 100 ~ 105 °C reaction, after the detection of the reaction, the reaction solution was reduced to room temperature, add K 3 PO 4 (33.96g, 0.16mol) purified water (110ml) solution, 2-(4-bromophenyl)-2-methylpropionate methyl ester (SM-2,51.42g, 0.20mol), temperature control 95~ 100 °C reaction, after the detection of the reaction, filtration, the filtrate was added to purified water (500ml), ethyl acetate (200ml×3) extraction, combined organic phase, anhydrous sodium sulfate dried, filtered, filtrate decompression concentration to dry intermediate I-2, Yield 86.6%, HPLC purity 99.66%.
Embodiment 3
[0088] Under argon protection at room temperature, compoundSY-1 (37.83g, 0.10mol), pinnacol biborate (25.39g, 0.1 mol), K 2 CO 3 (22.11g,0.16mol)、Pd(PPh 3 ) 4(5.78g, 5.0mmol) added to dimethyl sulfoxide (250ml), temperature control 105 ~ 110 °C reaction, after the detection of the reaction, the reaction solution dropped to room temperature, addED KOAc (15.70g, 0.16mol) purified water (110ml) solution, 2-(4-bromophenyl)-2-methylpropionate methyl ester (SM-2,38.57g, 0.15mol), temperature control 95 ~ 100 °C reaction, after the detection of the reaction, filtered, The filtrate was added to purified water (500ml), ethyl acetate (200ml×3) was extracted, combined with the organic phase, anhydrous sodium sulfate dried, filtered, and the filtrate was concentrated to dry after the intermediate I-2 was prepared, with a yield of 82.5% and a PURITy of 99.32% hplc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com