PDSS2 pathogenic mutant gene of nephrotic syndrome patient and detection reagent of PDSS2 pathogenic mutant gene
A technology for nephrotic syndrome and mutated genes, which is applied in the field of genetic disease gene detection, can solve the problems of long sequencing time, high detection cost, high cost, etc., and achieves the effect of low price and reduced economic burden.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Symptoms of patients with nephrotic syndrome PDSS2 pathogenic mutation gene and discovery of pathogenic mutation gene in patients with nephrotic syndrome of the present invention
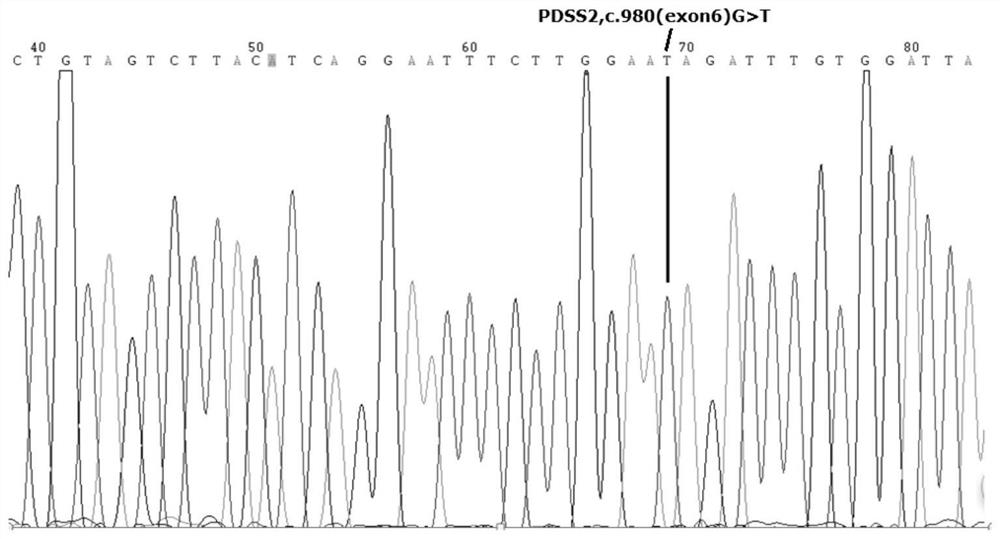
[0028] In a long-term clinical study, the inventor found a patient from the Nephrology Department of Xinqiao Hospital in a core consanguineous family with symptoms: proteinuria, hematuria, thin basement membrane, and a suspected NS patient. Through full sequence sequencing and comparison, the PDSS2 pathogenic mutation gene of the patient with nephrotic syndrome of the present invention is found.
[0029] The original sequence of PDSS2 gene is shown in SEQ ID NO.3. in,
[0030] ATAAATTCTGATGTCCAGCCTTTTATTAAAGAAAAGACCAGTGACTCCATGACTTTTAATCTAAACTCAGCTCCTGTAGTCTTACATCAGGAATTTCTTGGAA G The AGATTTGTGGGATTAAACAGATCGGAGAG part is the sixth exon, and this sequence includes the mutant gene site c.980(exon6)G of the present invention.
Embodiment 2
[0031] Example 2 Design and synthesis of amplification primers for the PDSS2 pathogenic mutation gene region of the present invention
[0032] The PDSS2 genome sequence was obtained from the NCBI database, and Primer software was used to design the detection primers. The primer sequences used are as follows. Primers were synthesized from Beijing Liuhe Huada Gene Co., Ltd. and purified by PAGE.
[0033] Amplification primers for PDSS2 gene mutation region
[0034] P1: TTCTGATGTCCAGCCTTTTTAT (SEQ ID NO. 1)
[0035] P2: TTAGCATTCACCACAGGCACT (SEQ ID NO. 2)
[0036] Sanger sequencing primer: P1.
Embodiment 3
[0037] Example 3: Detection reagent for PDSS2 pathogenic mutation gene of the present invention, and detection and verification of pathogenic mutation gene
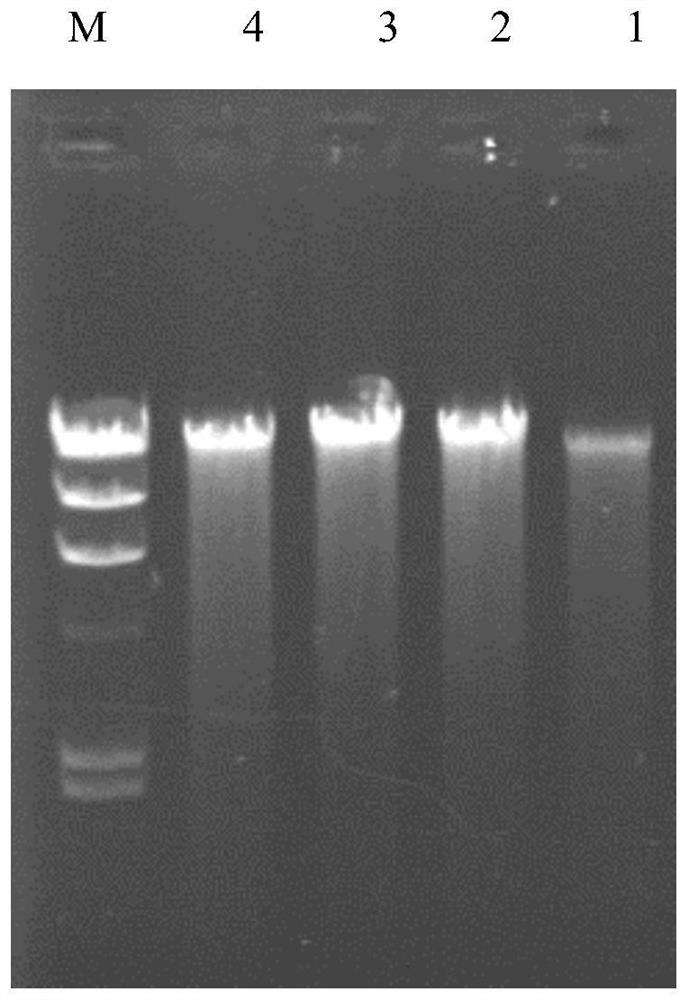
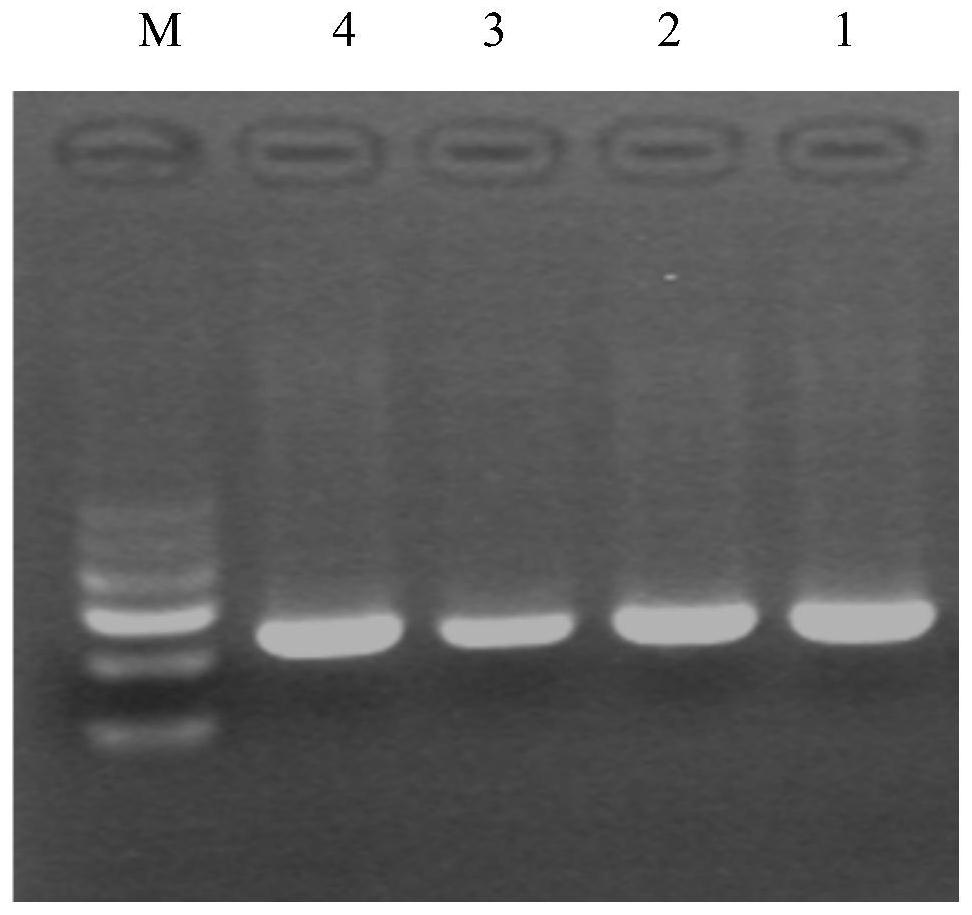
[0038] 1. Obtain clinical samples and set up experimental and control groups
[0039] 1) Experimental group
[0040] Patient 1, the sample was from the Nephrology Department of Xinqiao Hospital, symptoms: proteinuria, hematuria, thin basement membrane, suspected NS patient.
[0041] Patient 2, the sample was from the Nephrology Department of Xinqiao Hospital, symptoms: proteinuria, hematuria, thin basement membrane, suspected NS patient.
[0042] 3) Control group
[0043] Two normal subjects were selected as the control group, with sample numbers: 3 and 4.
[0044] 2. Experimental scheme and steps
[0045] 1. Screening test:
[0046] The diagnostic reagent of the present invention includes 1) a tester's sample genomic DNA extraction kit; 2) a PCR amplification kit: using primers P1 and P2 to amplify the tester's geno...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com