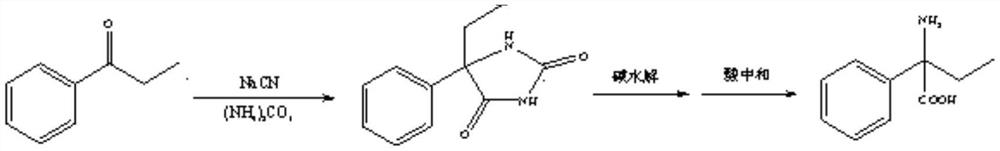
Preparation method of 2-amino-2-phenylbutyric acid
A technology of phenylbutyric acid and amino, which is applied in the field of preparation of trimebutine pharmaceutical intermediate 2-amino-2-phenylbutyric acid, can solve problems affecting product quality and yield, and achieve high atom economy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
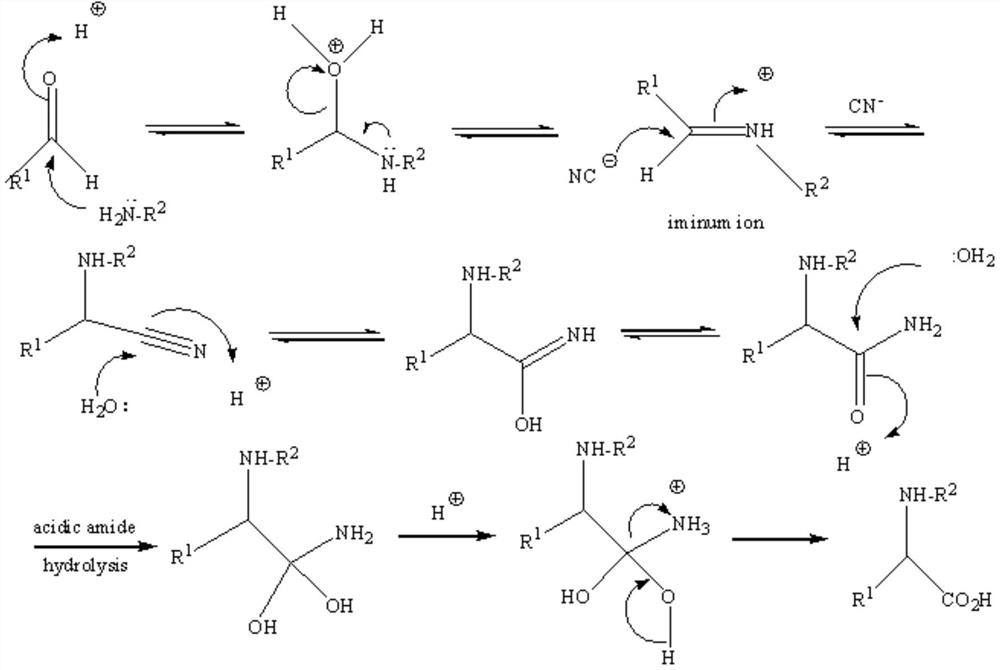
Image
Examples
Embodiment 1
[0038] 134g (1.0mol) of propiophenone, 245g (1.5mol) of 30% sodium cyanide solution, 192.16g (2.0mol) of ammonium carbonate and 6.7g of tetrabutylammonium bromide were added to a 2000mL there-necked flask, and then added 200g deionized water, stir to dissolve. The reaction solution was slowly heated to 65-70° C. and incubated for 15 hours. After the reaction was completed, suction filtration was performed, and the filter cake was washed with deionized water to obtain a wet product of 5-ethyl-5-phenylhydantoin.
[0039] After stirring and dissolving 5-ethyl-5-phenylhydantoin wet product, 120g (3.0mol) solid sodium hydroxide and 1000g deionized water, transfer to the autoclave, slowly heat up to 180°C, and heat preservation reaction 6h. After the reaction, the temperature was lowered to room temperature, the material was transferred to a 2000 mL flask, and hydrochloric acid was added dropwise to adjust pH=5.5-6.0. The material was extracted, the filter cake was washed with dei...
Embodiment 2
[0041] 134g (1.0mol) of propiophenone, 294g (1.8mol) of 30% sodium cyanide solution, 288.24g (3.0mol) of ammonium carbonate and 6.7g of benzyltriethylammonium chloride were added to the there-necked flask of 2000mL, Add 300 g of deionized water and stir to dissolve. The reaction solution was slowly heated to 75-80° C. and incubated for 10 hours. After the reaction was completed, suction filtration was performed, and the filter cake was washed with deionized water to obtain a wet product of 5-ethyl-5-phenylhydantoin.
[0042] The above-mentioned 5-ethyl-5-phenylhydantoin wet product, 120g (3.0mol) solid sodium hydroxide and 1000g deionized water were stirred and dissolved and then transferred to the autoclave, slowly warming up to 180° C. Reaction 6h. After the reaction, the temperature was lowered to room temperature, the material was transferred to a 2000 mL flask, and 50% sulfuric acid was added dropwise to adjust pH=5.5-6.0. The material was extracted, the filter cake was...
Embodiment 3
[0044] 134g (1.0mol) of propiophenone, 294g (1.8mol) of 30% sodium cyanide solution, 480.4g (5.0mol) of ammonium carbonate and 13.4g of tetrabutylammonium bromide were added to a 2000mL there-necked flask, and then added 500g deionized water, stir to dissolve. The reaction solution was slowly heated to 65-70° C. and incubated for 15 hours. After the reaction was completed, suction filtration was performed, and the filter cake was washed with deionized water to obtain a wet product of 5-ethyl-5-phenylhydantoin.
[0045]The above-mentioned 5-ethyl-5-phenylhydantoin wet product, 120g (3.0mol) solid sodium hydroxide and 1000g deionized water were stirred and dissolved, and then transferred to the autoclave, slowly warming up to 160 ° C, insulation The reaction was carried out for 8 hours. After the reaction, the temperature was lowered to room temperature, the material was transferred to a 1000 mL flask, and 50% sulfuric acid was added dropwise to adjust pH=5.5-6.0. The material...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com