Novel aza-crown ether compound as well as cationic liposome, preparation method and application thereof
A cationic liposome and azacrown ether technology, applied in the field of biomedicine, can solve problems such as clinical difficulties, high cytotoxicity, and unsatisfactory cell transfection efficiency, and achieve broad application range, low cytotoxicity, and reduction of multidrugs The effect of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
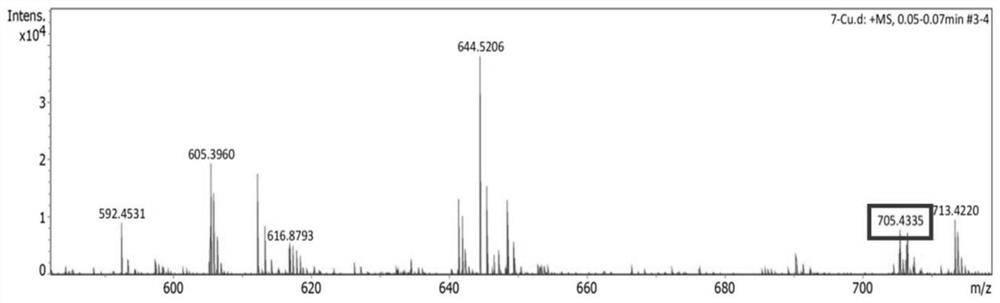
[0044] Example 1: Preparation and detection of novel azacrown ether compounds
[0045] 1. Experimental method
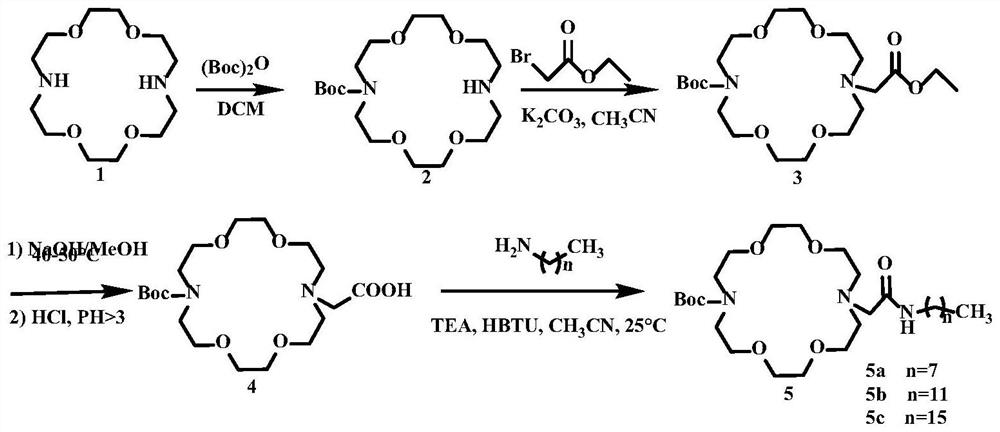
[0046] 1) Preparation of 1,4,10,13-tetraoxa-7,16-diazacyclooctadecane, the synthetic route is as follows figure 1 shown
[0047] 1,2-bis(2-chloroethoxy)ethane (19.03g, 101.7mmol) and sodium iodide (33.4g, 222.8mmol) were added to acetone (50mL), stirred and refluxed for 72h; cooled to room temperature and extracted The filtrate was filtered, and the filtrate was spin-dried to obtain a light yellow oily viscous residue. The above residue was dissolved in methyl tert-butyl ether (MTBE), and anhydrous sodium sulfate (60.3 g, 240 mL) was used as the aqueous phase, and the residue was left alone. Set the liquid separation, take the MTBE phase of the upper layer, spin the MTBE phase to dryness, and obtain the intermediate product, 1,2-bis(2-iodoethoxy)ethane as a colorless oily liquid;
[0048] 1,8-Diamino-3,6-dioxoctane (32.46 g, 219.0 mmol) was dissolved in acetonitri...
Embodiment 2
[0072] Example 2: Preparation of Cationic Liposomes
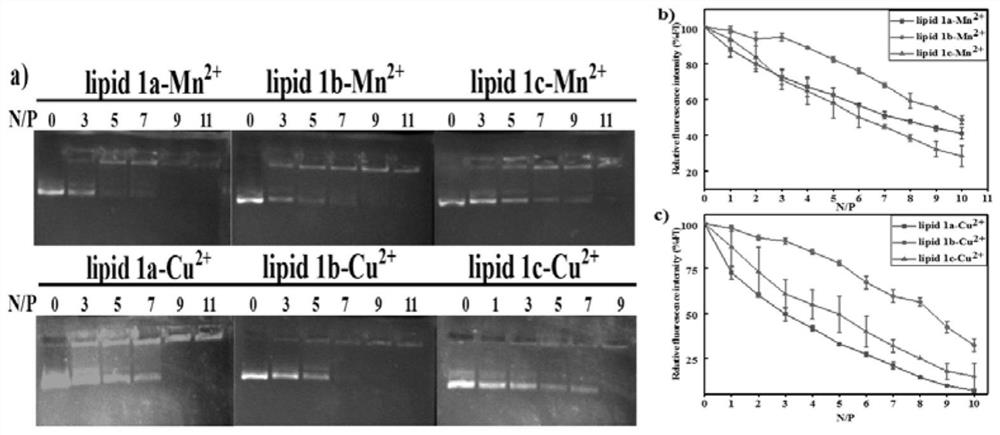
[0073] The neutral lipid dioleoylphosphatidylethanolamine (0.005mmol) was combined with the novel azacrown ether compounds, namely 5a, 5b, and 5c, respectively, in chloroform, dried under reduced pressure with nitrogen, and the chloroform solvent was removed. MnSO for lipid membrane 4 ·H 2 O(5mL, 1mM) and Cu(NO 3 ) 2 ·3H 2 The O (5 mL, 1 mM) solution was hydrated to a final lipid concentration of 1 mM. The mixture was stirred until the film was completely resuspended and then sonicated (60°C) in a cell disintegrator to give cationic liposome lipid 1-M (1=1a, 1b, 1c, M=Mn 2+ and Cu 2+ ), stored at 4°C.
Embodiment 3
[0074] Example 3: Preparation of cationic liposome / pDNA complexes (lipoplexes)
[0075] 0ul, 1.050ul, 1.125ul, 1.875ul, 2.625ul, 3.375ul and 4.125ul of cationic lipid were thoroughly mixed with 0.125ug of DNA at N / P ratios of 0, 1, 3, 5, 7, 9 and 11, respectively , and incubated at room temperature for 30 min to obtain lipid complexes. The theoretical N / P ratio represents the charge ratio (molar ratio) of cationic lipid to nucleotide base, and takes into account an average nucleotide mass of 330 g / mol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com