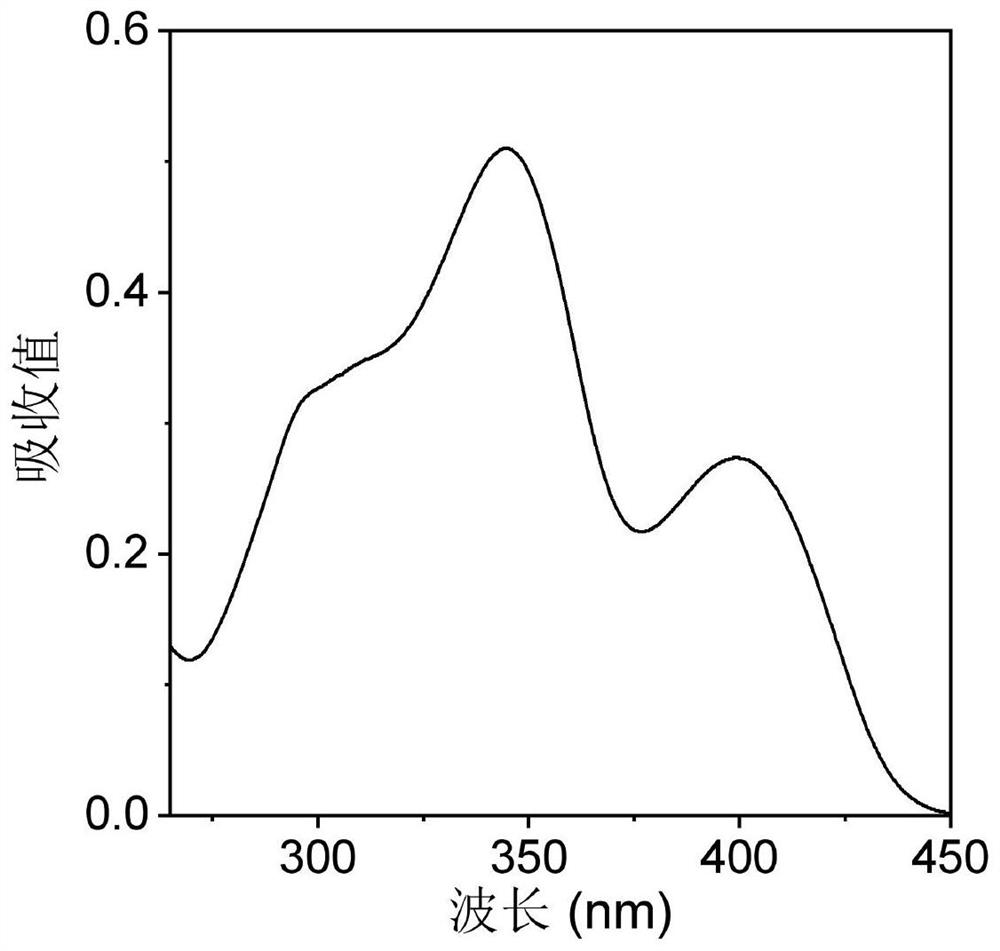
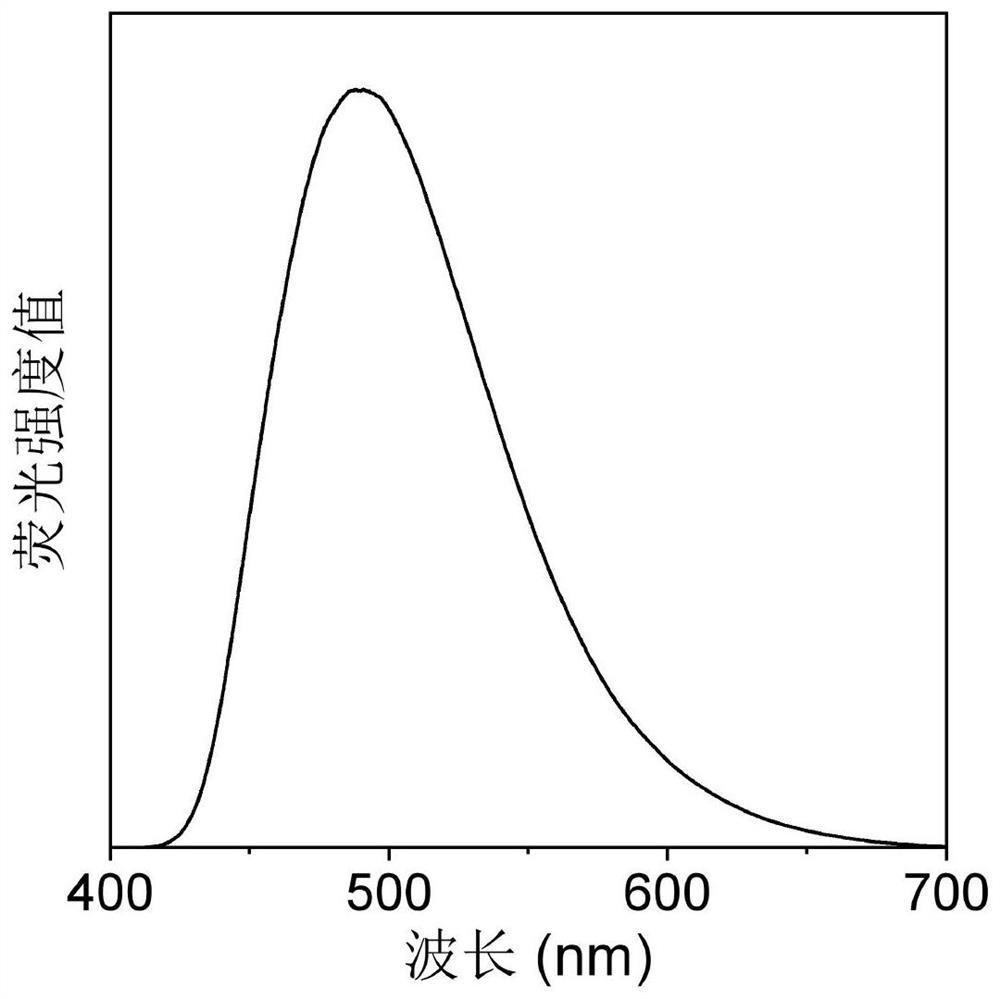
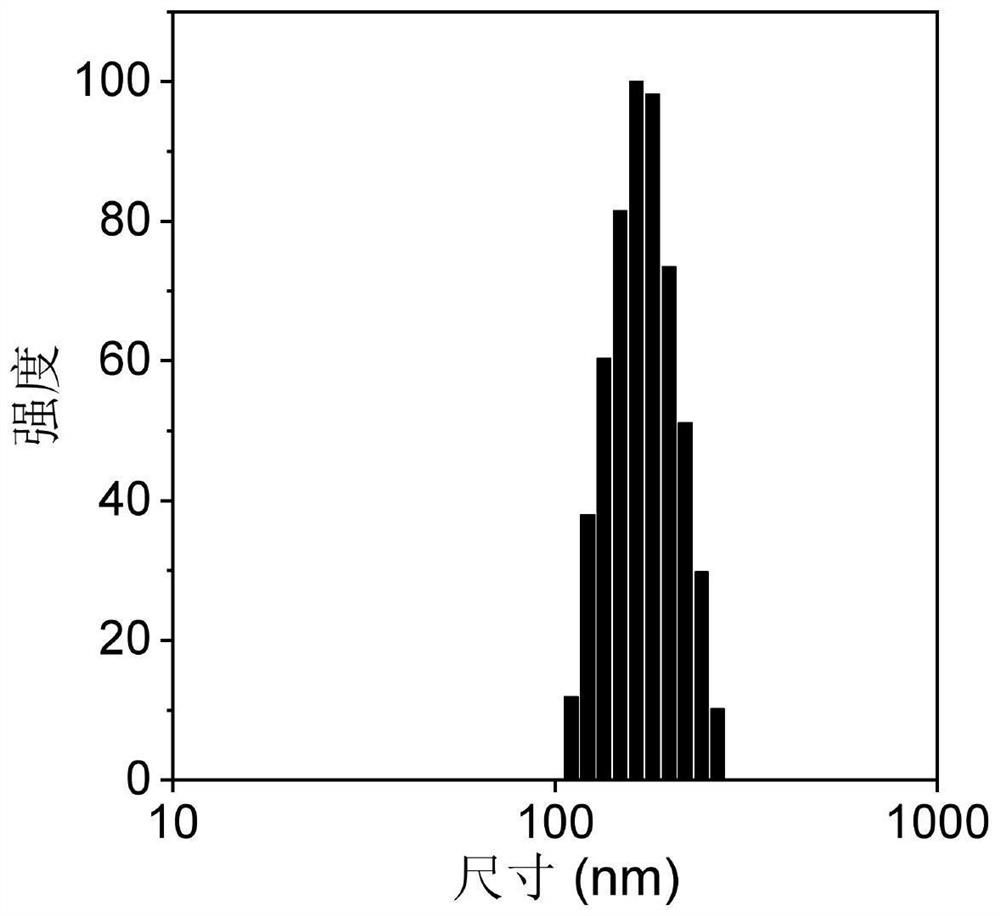
Photosensitizer with fluorescence imaging and photodynamic gram-positive bacterium killing activity as well as preparation method and application of photosensitizer
A Gram-positive bacteria, fluorescence imaging technology, applied in photodynamic therapy, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problem of few photosensitizers, achieve good luminescence ability, strong reactive oxygen species Generating ability, efficient photodynamic killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] In the present invention, when the photosensitizer has the structure shown in formula I, the preparation method comprises the following steps:
[0053] (1-1) The compound having the structure shown in formula III is mixed with 3,6-dichloropyridazine, a palladium catalyst and an inorganic base to carry out Suzuki reaction to obtain the compound having the structure shown in formula IV;
[0054]
[0055] (1-2) The compound having the structure represented by the formula IV and N,N-dimethylethylenediamine are mixed to carry out a substitution reaction to obtain the compound having the structure represented by the formula V.
[0056]
[0057] (1-3) Mixing the compound with the structure of formula V and methyl iodide to carry out a salt-forming reaction to obtain a photosensitizer with the structure of formula I.
[0058] In the present invention, the compound with the structure shown in formula III is mixed with 3,6-dichloropyridazine, a palladium catalyst, an inorga...
Embodiment 1
[0085] To prepare the photosensitizer with the structure of formula II where R is hydrogen, the synthetic route is as follows, the raw materials are denoted as compound 1 to compound 4 according to the labels in the synthesis route, and the product is denoted as DAPO-O:
[0086]
[0087] (1) Compound 1 (1 g, 6 mmol), compound 2 (2.67 g, 7.2 mmol), [1,1'-bis(diphenylphosphonium)ferrocene]dichloropalladium dichloromethane complex ( 245mg, 0.3mmol), potassium phosphate (3.82g, 18mmol) and tetrahydrofuran were mixed, refluxed at 65°C under the protection of nitrogen, and the reaction progress was monitored with TLC plate until compound 1 disappeared completely, and the obtained reaction solution was mixed with saturated aqueous ammonium chloride solution. Methylene chloride was mixed for extraction, the organic phase was separated, and the aqueous phase was extracted twice with dichloromethane. The organic phases were combined and purified by silica gel column chromatography. Th...
Embodiment 2
[0129] To prepare a photosensitizer with the structure shown in formula II where R is a methoxy group, other conditions are the same as those in Example 1, except that compound 2 is replaced with the following compound:
[0130]
[0131] The NMR analysis of the final product shows that the structure of the final product is as follows:
[0132]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com