Method for recovering rubidium and cesium in salt lake brine by using heteropolyacid salt electrode
A technology for heteropoly acid salt and salt lake brine, applied in the field of separation and recovery of valuable metals in salt lake brine, can solve problems such as long time consumption, and achieve the effects of improving separation and recovery efficiency, low cost and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
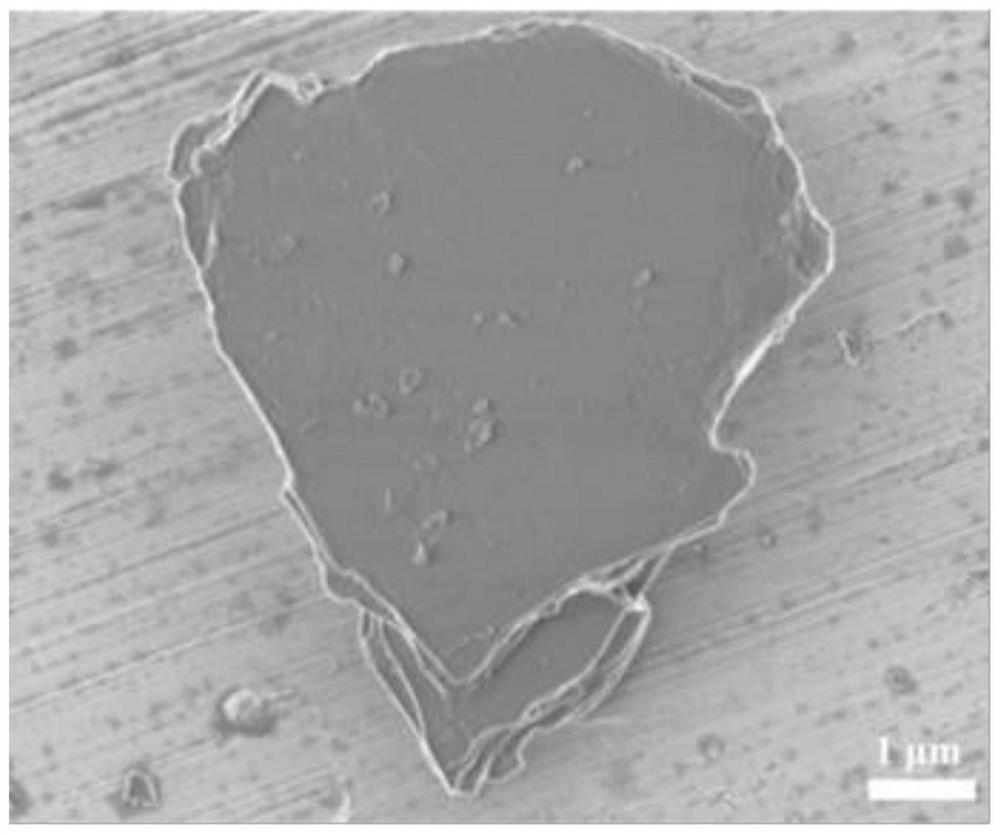
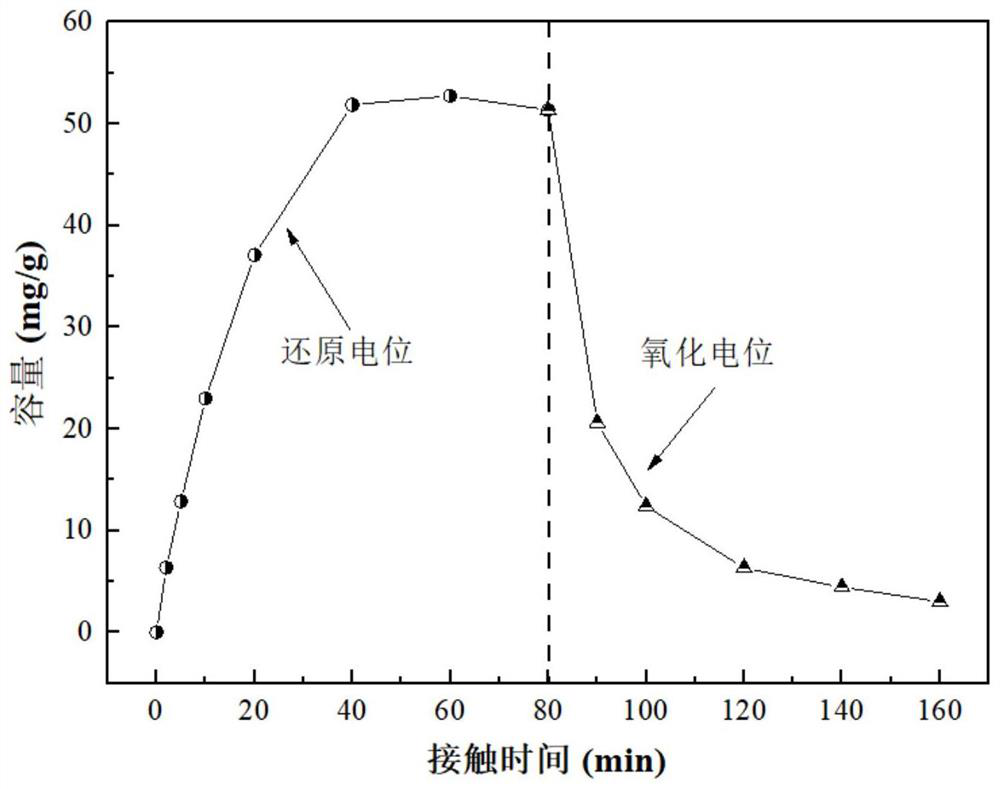
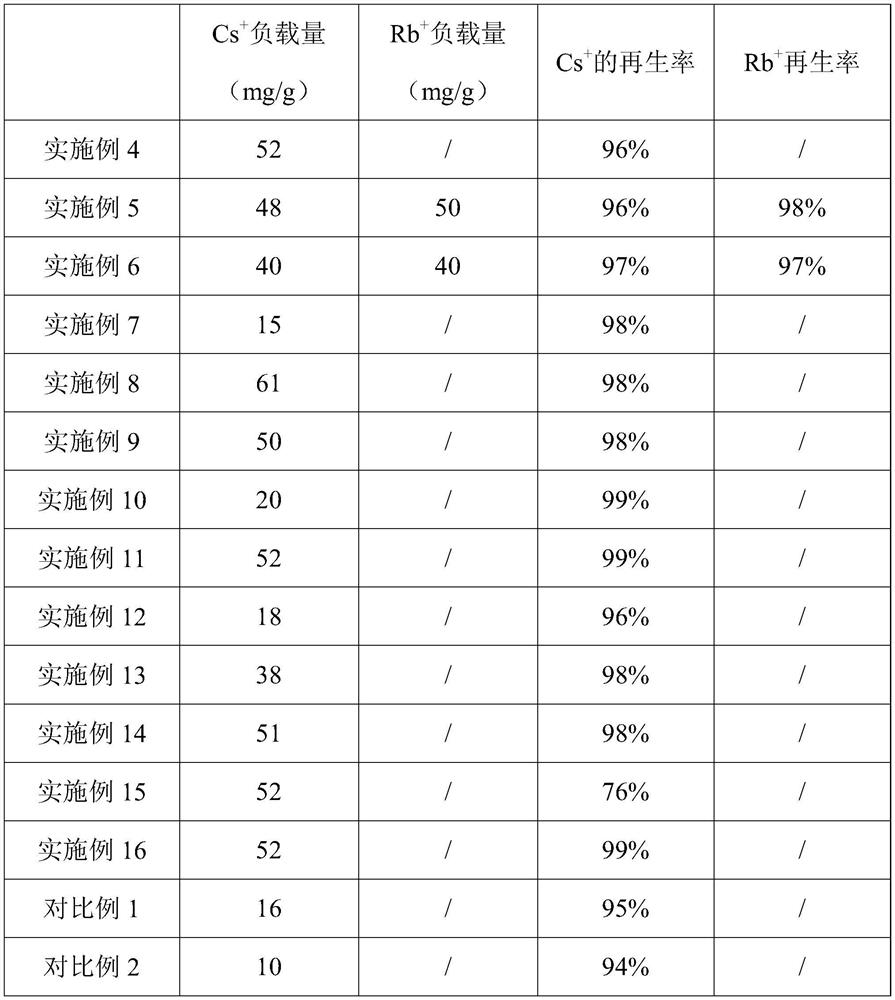
[0057] This embodiment provides a method for preparing a heteropolyacid salt molecular sieve and a heteropolyacid salt electrode thereof. The method for preparing a heteropolyacid salt molecular sieve includes: mixing 28.47g of sodium silicate nonahydrate, 8.02g of sodium chloride, 3.016g of Mix g sodium hydroxide with 65 mL deionized water to obtain mixed solution A; mix 9.45 g of oxalic acid with 25 mL of deionized water, and then add 1.83 g of sodium metavanadate to obtain mixed solution B; add mixed solution B to mixed solution A , mixed and stirred for 1 h, aged for 24 h, and then hydrothermally reacted at 180 ° C for 36 h, filtered, washed and dried to obtain sodium vanadyl silicate.
[0058] The preparation method of the heteropolyacid salt electrode includes: stirring and mixing sodium vanadyl silicate, polyvinylidene fluoride and dimethylformamide, and the mass ratio of the sodium vanadyl silicate and polyvinylidene fluoride is 1:0.125, The obtained slurry is coated o...
Embodiment 2
[0060] This embodiment provides a method for preparing a heteropolyacid salt molecular sieve and a heteropolyacid salt electrode thereof. The method for preparing a heteropolyacid salt molecular sieve includes: mixing 28.47g of sodium silicate nonahydrate, 8.02g of sodium chloride, 3.016g of Mix g sodium hydroxide with 65 mL deionized water to obtain mixed solution A; mix 9.45 g of oxalic acid with 25 mL of deionized water, and then add 1.75 g of sodium metavanadate to obtain mixed solution B; add mixed solution B to mixed solution A , mixed and stirred for 1 h, aged for 4 h, and then hydrothermally reacted at 230 ° C for 24 h, filtered, washed and dried to obtain sodium vanadiosilicate.
[0061] The preparation method of the heteropolyacid salt electrode comprises: stirring and mixing sodium vanadyl silicate, polyvinylidene fluoride and dimethylformamide, and the mass ratio of the sodium vanadyl silicate and polyvinylidene fluoride is 1:0.1, The obtained slurry is coated on t...
Embodiment 3
[0063] This embodiment provides a method for preparing a heteropolyacid salt molecular sieve and a heteropolyacid salt electrode thereof. The method for preparing a heteropolyacid salt molecular sieve includes: mixing 28.47g of sodium silicate nonahydrate, 8.02g of sodium chloride, 3.016g of Mix g sodium hydroxide with 65 mL deionized water to obtain mixed solution A; mix 9.45 g of oxalic acid with 25 mL of deionized water, and then add 2.73 g of vanadium pentoxide to obtain mixed solution B; add mixed solution B to mixed solution A , mixed and stirred for 1 h, aged for 30 h, and then hydrothermally reacted at 200 ° C for 30 h, filtered, washed and dried to obtain sodium vanadyl silicate.
[0064] The preparation method of the heteropolyacid salt electrode comprises: stirring and mixing sodium vanadyl silicate, polyvinylidene fluoride and dimethylformamide, and the mass ratio of the sodium vanadyl silicate and polyvinylidene fluoride is 1:0.15, The obtained slurry is coated on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com